Introduction

Are you a driven clinical research professional with a vision for leadership? Do you aspire to orchestrate the complex, life-saving processes that bring new medical therapies from concept to reality? If you're aiming for the pinnacle of clinical trial management, the role of Director of Clinical Operations offers not only immense professional fulfillment but also a substantial financial reward. This position is the strategic heart of clinical development, demanding a rare blend of scientific knowledge, business acumen, and inspirational leadership.
The financial compensation for this critical role reflects its importance. A Director of Clinical Operations can expect to earn an average base salary well into the six figures, with total compensation packages often exceeding $200,000 to $250,000 or more, especially for those with specialized expertise and a proven track record. This is more than just a job; it's a capstone career for those dedicated to advancing human health.
I recall a conversation years ago with a senior director at a leading biotech firm. She wasn't just managing timelines and budgets; she was stewarding hope. "Every line in my spreadsheet," she said, "represents a family waiting for a breakthrough. My job is to clear the path so science can get to them faster." It was a profound reminder that behind the immense responsibility and impressive salary lies a deeply human purpose.
This comprehensive guide will illuminate every facet of the Director of Clinical Operations career path. We will dissect the salary you can expect, explore the factors that drive it higher, map out your long-term career trajectory, and provide a step-by-step roadmap to get you there.
### Table of Contents
- [What Does a Director of Clinical Operations Do?](#what-they-do)
- [Average Director of Clinical Operations Salary: A Deep Dive](#salary-deep-dive)
- [Key Factors That Influence Salary](#key-factors)
- [Job Outlook and Career Growth](#job-outlook)
- [How to Get Started in This Career](#how-to-start)
- [Conclusion](#conclusion)
---
What Does a Director of Clinical Operations Do?

A Director of Clinical Operations (DCO) is a senior-level executive responsible for the strategic planning, execution, and oversight of all clinical trial activities within a pharmaceutical company, biotechnology firm, or Contract Research Organization (CRO). They are the master conductors of the clinical research orchestra, ensuring that every section—from site selection to data analysis—plays in perfect harmony to meet regulatory standards, stay within budget, and adhere to strict timelines.
This role is not about managing a single trial but about managing the entire clinical trial *portfolio* and the infrastructure that supports it. The DCO ensures that the company's operational capabilities are robust enough to successfully execute its clinical development plan. They bridge the gap between high-level corporate strategy and the day-to-day, on-the-ground reality of running clinical studies.
Core Responsibilities Include:
- Strategic Leadership: Developing and implementing the overall clinical operations strategy in alignment with the company's research and development goals. This involves long-range planning, resource allocation, and setting departmental objectives.
- Operational Oversight: Managing all phases of clinical trials (Phase I-IV), from protocol development and site feasibility to patient recruitment, study closeout, and the final clinical study report (CSR).
- Financial Management: Developing and managing multi-million dollar departmental and clinical trial budgets. This includes forecasting expenses, negotiating contracts with vendors and clinical sites, and ensuring financial accountability across all projects.
- Team Leadership and Development: Building, mentoring, and leading a team of clinical operations professionals, which may include Clinical Trial Managers (CTMs), Clinical Research Associates (CRAs), and Clinical Trial Assistants (CTAs). They foster a culture of excellence, compliance, and continuous improvement.
- Vendor and CRO Management: Selecting, qualifying, and managing relationships with external vendors, including Contract Research Organizations (CROs), central laboratories, and data management providers. The DCO ensures these partners meet quality standards and deliver on their contractual obligations.
- Regulatory Compliance: Ensuring all clinical trial activities are conducted in strict adherence to Good Clinical Practice (GCP), ICH guidelines, and regulations from bodies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency).
- Process Improvement: Continuously evaluating and optimizing clinical operations processes and systems (e.g., SOPs, clinical trial management systems) to enhance efficiency, quality, and speed.
---
### A "Day in the Life" of a Director of Clinical Operations
While no two days are identical, a typical day blends strategic meetings with hands-on problem-solving:
- 8:30 AM - Morning Leadership Huddle: The day begins with a meeting with the C-suite (e.g., Chief Medical Officer) or VPs of Development to discuss high-level program status, key risks, and strategic priorities for the week.
- 9:30 AM - Program Review Meeting: Lead a cross-functional meeting with a team of Clinical Trial Managers. The agenda covers patient recruitment metrics for a pivotal Phase III oncology trial, a potential protocol deviation at a key site in Europe, and a review of the data-cleaning timeline.
- 11:00 AM - Vendor Governance Call: A quarterly business review with a primary CRO partner. The DCO reviews performance metrics, discusses budget variances, and strategizes on how to accelerate enrollment for a slow-moving study.
- 12:30 PM - Lunch & Mentoring: A working lunch with a newly promoted Associate Director, providing coaching on managing difficult site relationships and developing their leadership skills.
- 2:00 PM - Budget Forecasting Session: Deep dive into spreadsheets with the finance department to model costs for a newly planned Phase II study, factoring in everything from site activation fees to drug supply logistics.
- 3:30 PM - Regulatory Strategy Session: Meet with the Regulatory Affairs team to discuss feedback from the FDA on a recent submission and plan the operational execution of a requested post-marketing study.
- 4:30 PM - Head-Down Time: Finally, a chance to respond to critical emails, review and approve new departmental Standard Operating Procedures (SOPs), and prepare for tomorrow's board presentation.
The role is demanding, dynamic, and requires the ability to seamlessly switch between a 30,000-foot strategic view and a 3-foot view of a critical operational detail.
---
Average Director of Clinical Operations Salary: A Deep Dive
The Director of Clinical Operations salary is a reflection of the immense responsibility and specialized expertise the role demands. Compensation is not just a simple base salary; it's a comprehensive package that often includes significant variable pay, making the total earning potential exceptionally high.
According to data from leading salary aggregators (accessed in Q2 2024), the compensation landscape looks like this:
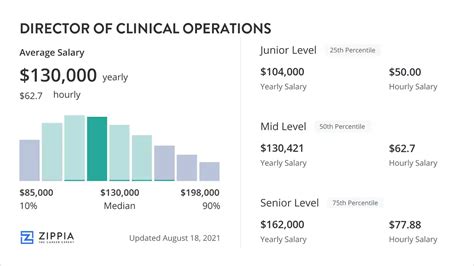
- Salary.com reports the median base salary for a Director of Clinical Operations in the United States is $191,959, with a typical range falling between $166,801 and $222,058.
- Glassdoor indicates a total pay average of $216,423 per year, combining a base salary average of $181,879 with approximately $34,544 in additional pay (bonuses, stock, etc.).
- Payscale.com shows an average base salary of $169,573, with a reported range from $123,000 to $216,000. They also note that bonuses can reach up to $49,000 and profit sharing up to $30,000 for some individuals.
Aggregating these sources, we can establish a reliable picture:
- National Average Base Salary: Approximately $170,000 - $190,000
- Typical Base Salary Range: $150,000 - $225,000
- Typical Total Compensation Range (with bonuses): $180,000 - $275,000+
It's important to note that the most experienced directors working in high-cost-of-living biotech hubs for large, profitable pharmaceutical companies can see their total compensation packages soar well above $300,000 when long-term incentives like stock options are factored in.
### Salary by Experience Level
Salary growth in this field is significant and directly tied to experience. A professional's journey to the Director level involves years of progressive responsibility, and compensation rises steeply at each stage.
| Career Stage | Typical Title(s) | Years of Experience | Average Base Salary Range | Typical Total Compensation Range |
| :--- | :--- | :--- | :--- | :--- |
| Entry-Level (on the path) | Clinical Trial Assistant (CTA), Clinical Research Coordinator (CRC) | 0-3 | $50,000 - $75,000 | $55,000 - $80,000 |
| Mid-Career (Pre-Director) | Clinical Research Associate (CRA), Clinical Trial Manager (CTM) | 3-10 | $90,000 - $150,000 | $100,000 - $180,000 |
| Senior/Lead (Pre-Director) | Senior CTM, Associate Director of Clinical Operations | 8-15 | $150,000 - $180,000 | $170,000 - $220,000 |
| Director Level | Director of Clinical Operations | 10-15+ | $170,000 - $225,000 | $190,000 - $275,000+ |
| Senior Director / VP Level | Senior Director, Vice President of Clinical Operations | 15+ | $220,000 - $300,000+ | $275,000 - $500,000+ |
*Note: These are national averages and can vary significantly based on the factors discussed in the next section.*
### Understanding Total Compensation
A DCO's paycheck is far more than just their base salary. To truly understand the earning potential, you must consider the entire compensation package.
- Annual Bonus: This is the most common form of variable pay. It's typically performance-based, tied to both individual and company goals (e.g., meeting enrollment targets, successful study completion, positive regulatory milestones). A typical bonus for a Director might range from 15% to 30% of their base salary. For a director earning $190,000, this translates to a bonus of $28,500 to $57,000.
- Stock Options / Restricted Stock Units (RSUs): Particularly prevalent in publicly traded pharmaceutical/biotech companies, this is a form of long-term incentive (LTI). You are granted equity in the company that vests over several years. This can be an incredibly lucrative part of the package, especially if the company's stock performs well. For pre-IPO or startup biotech firms, stock options represent a higher-risk, higher-reward component that could be life-changing if the company is successful.
- Profit Sharing: Some companies offer a profit-sharing plan where a portion of the company's annual profits is distributed to employees. This is more common in established, profitable corporations.
- Sign-On Bonus: To attract top talent, companies often offer a significant one-time sign-on bonus, which can range from $10,000 to $50,000 or more, to compensate for forfeited bonuses or unvested equity at a previous job.
- Comprehensive Benefits: Beyond direct pay, the value of the benefits package is substantial. This includes premium health, dental, and vision insurance; a strong 401(k) matching program (often 4-6% of salary); generous paid time off (PTO); and tuition reimbursement for further education or certifications.
When evaluating a job offer, a savvy candidate looks beyond the base salary to calculate the "total rewards" of the position, as the variable and long-term components can dramatically increase overall earnings.
---
Key Factors That Influence Director of Clinical Operations Salary

While we've established a national average, a DCO's actual salary is a dynamic figure influenced by a confluence of critical factors. Understanding these variables is key to negotiating the best possible compensation and strategically planning your career moves. This is the most crucial section for anyone looking to maximize their earning potential in this field.
### 1. Level of Education and Certifications
Your educational background provides the foundational knowledge for this scientific and managerial role. While experience often trumps education later in a career, your degree directly impacts your entry point and long-term ceiling.
- Bachelor's Degree: A Bachelor of Science (BS) in a life science discipline (e.g., Biology, Chemistry, Nursing, Pharmacy) is the standard minimum requirement. Professionals with only a BS will need extensive, high-quality experience (12-15+ years) to reach the Director level.
- Master's Degree: This is often the sweet spot and a significant differentiator. A Master's degree can accelerate your path to leadership. Highly relevant degrees include:
- Master of Science (MS) in Clinical Research or Regulatory Affairs: Directly applicable and highly valued.
- Master of Business Administration (MBA): Demonstrates strong business and financial acumen, crucial for budget management and strategic planning. An MBA combined with a science undergrad is a powerful combination.
- Master of Public Health (MPH) or Master of Health Administration (MHA): Excellent for understanding the broader healthcare landscape.
- *Salary Impact:* Holding a relevant Master's degree can command a 10-15% salary premium over a candidate with only a Bachelor's degree and similar experience.
- Doctoral Degree (PhD, PharmD, MD): This represents the highest level of formal education and commands the highest salaries. These individuals bring deep scientific or medical expertise. A PhD can be invaluable in complex therapeutic areas like oncology or immunology. A PharmD is perfectly suited for pharmaceutical development. An MD who transitions to an industry operations role is rare and highly sought after, often taking on roles like Medical Director before moving into operations leadership.
- *Salary Impact:* Professionals with a doctorate, particularly an MD or PharmD, often start at a higher level (e.g., Associate Director or Director) and can command salaries at the very top of the pay scale, easily exceeding the $225,000+ base mark.
- Professional Certifications: While not a substitute for degrees or experience, certifications demonstrate a commitment to the profession and specialized knowledge.
- Project Management Professional (PMP): Highly respected and signals expertise in managing complex projects, timelines, and budgets.
- Association of Clinical Research Professionals (ACRP-CP): A general certification for the clinical research field.
- Society of Clinical Research Associates (SOCRA) Certification (CCRP): Another well-regarded industry certification.
- *Salary Impact:* Certifications like the PMP can add 5-10% to a salary offer and make a resume stand out in a competitive applicant pool.
### 2. Years and Quality of Experience
This is, without a doubt, the single most important factor. The journey to DCO is a marathon, not a sprint, and each stage builds the necessary skills and commands a higher salary.
- 0-5 Years (Foundation Building): Starting as a Clinical Research Coordinator (CRC) at a hospital or a Clinical Trial Assistant (CTA) at a CRO/Sponsor. You learn the nuts and bolts of trial execution. Salary: $50k - $85k.
- 5-10 Years (Management Track): Progressing to a Clinical Research Associate (CRA) role, involving travel and site management, and then to a Clinical Trial Manager (CTM) or Clinical Project Manager. This is where you gain your first taste of project-level leadership and budget responsibility. Salary: $90k - $150k.
- 10-15 Years (Senior Leadership): Excelling as a CTM leads to a Senior CTM or, more commonly, an Associate Director of Clinical Operations role. Here, you begin managing other managers and overseeing a program of multiple trials. You are now a senior leader, and your salary reflects this. Salary: $150k - $180k.
- 15+ Years (Director and Beyond): With a proven track record of successful program delivery, budget management, and team leadership, you are now a competitive candidate for Director roles. At this stage, the *quality* of your experience is paramount. Did you lead a successful NDA/BLA submission? Do you have experience in a highly competitive therapeutic area? Have you managed global trials? These achievements directly translate into higher salary offers. Salary: $170k - $275k+.
### 3. Geographic Location
Where you work has a massive impact on your salary, driven by the cost of living and the concentration of life sciences companies.
- Top-Tier Hubs: These locations are home to a dense ecosystem of pharmaceutical giants, established biotechs, venture-backed startups, and top-tier academic institutions. The intense competition for talent drives salaries significantly higher than the national average.
- San Francisco Bay Area, CA: The undisputed leader. Salaries here can be 25-40% above the national average.
- Boston/Cambridge, MA: A close second, another major biotech and pharma hub. Expect salaries 20-35% above average.
- San Diego, CA: A thriving life sciences cluster. Salaries are typically 15-25% above average.
- New York/New Jersey Corridor: The "Big Pharma" heartland. Salaries are 10-25% above average.
- Raleigh-Durham (Research Triangle Park), NC: A major hub for CROs and R&D, with salaries 5-15% above average.
- Mid-Tier Markets: Cities with a growing but less dense life sciences presence. Salaries here are often at or slightly above the national average. Examples include Philadelphia, PA; Seattle, WA; and the Washington D.C./Maryland area.
- Lower-Cost Areas: Regions with fewer industry employers will naturally have salaries closer to or below the national average. However, the lower cost of living can often offset the difference in pay.
- The Rise of Remote Work: The COVID-19 pandemic accelerated the trend of remote work. While many senior leadership roles still require a presence in a central office, more companies are open to remote directors. Remote salaries are often benchmarked to a national average or tiered based on the employee's location, but they provide access to opportunities outside of the traditional hubs.
| City/Region | Salary Index (vs. National Average) | Example Estimated Base Salary |
| :--- | :--- | :--- |
| San Francisco, CA | +35% | ~$255,000 |
| Boston, MA | +30% | ~$245,000 |
| San Diego, CA | +20% | ~$228,000 |
| New York, NY | +18% | ~$224,000 |
| Raleigh, NC | +10% | ~$209,000 |
| National Average | Base | ~$190,000 |
| Chicago, IL | -2% | ~$186,000 |
### 4. Company Type and Size
The type of organization you work for fundamentally shapes your role, responsibilities, and compensation structure.
- Large Pharmaceutical Companies ("Big Pharma"): (e.g., Pfizer, Johnson & Johnson, Merck)
- Pros: Highest base salaries, exceptional benefits, structured career paths, and job stability. They have massive budgets and run global mega-trials.
- Cons: Can be bureaucratic, slower-paced, and your individual impact might feel diluted.
- Salary: Top of the market for base pay and cash bonuses.
- Biotechnology Companies:
- Established/Profitable Biotech: (e.g., Amgen, Gilead, Vertex) Offer compensation packages that are highly competitive with Big Pharma, often with a significant equity component.
- Startup/Pre-IPO Biotech: This is a high-risk, high-reward environment. Base salaries might be slightly below the market rate, but this is compensated with a substantial grant of stock options. If the company's drug is successful or the company is acquired, this equity can be worth far more than the salary difference.
- Contract Research Organizations (CROs): (e.g., IQVIA, ICON, PPD/Thermo Fisher)
- Pros: Excellent training ground, exposure to a wide variety of therapeutic areas and sponsors, fast-paced environment.
- Cons: The work is client-service oriented, which can be demanding. Profit margins can be tighter than on the sponsor side.
- Salary: Base salaries are very competitive, often on par with pharma, as CROs need to attract top operational talent to win business. Bonuses are heavily tied to project profitability and client satisfaction.
- Medical Device Companies:
- The clinical trial process for devices can differ significantly from drugs/biologics. Directors in this space need expertise in 510(k) vs. PMA pathways.
- Salary: Generally competitive, but may be slightly less than top-tier pharma/biotech roles unless it's a highly innovative, high-growth device company.
### 5. Area of Specialization (Therapeutic Area)
While a DCO is an operational expert first, deep experience in a specific therapeutic area (TA) can make you a much more valuable and highly-paid asset. TAs that are complex, highly competitive, and have a large market potential command the highest salaries.
- Top-Tier TAs:
- Oncology/Immuno-Oncology: The most complex, fast-moving, and well-funded area in drug development. Directors with extensive oncology trial experience (especially cell and gene therapy) are in extremely high demand and can command a significant salary premium.
- Rare Diseases/Orphan Drugs: These trials have unique challenges (e.g., finding patients, specialized endpoints). Expertise here is niche and highly valued.
- CNS (Central Nervous System): Diseases like Alzheimer's and Parkinson's are notoriously difficult to study. A proven track record in this area is a major differentiator.
- Strong, Stable TAs:
- Cardiology, Infectious Diseases, Vaccines, and Immunology are always active areas with robust pipelines.
- Less Complex TAs:
- Areas like Dermatology or certain metabolic disorders might have more straightforward trial designs, and while still requiring expertise, may not command the same premium as oncology.
### 6. In-Demand Skills
Beyond the standard job description, certain skills are particularly potent salary boosters in today's market. Cultivating these will directly translate to better offers.
- Global Trial Management: Experience managing multi-regional trials across North America, Europe, and Asia-Pacific is a huge asset. It demonstrates an understanding of varying regulatory landscapes and cultural nuances.
- CRO/Vendor Partnership and Oversight: The ability to not just manage but truly *partner* with CROs to drive performance is critical. This includes strong negotiation, governance, and relationship management skills.
- Financial and Business Acumen: Directors who can speak the language of finance—accurately forecasting budgets, analyzing variances, and linking operational performance to financial outcomes—are seen as true business leaders.
- Decentralized Clinical Trial (DCT) Expertise: Knowledge of and experience with implementing remote/hybrid trial components (telemedicine, eConsent, wearables, direct-to-patient drug shipment) is a forward-looking skill that companies are actively seeking.
- Data-Driven Decision Making: The ability to leverage clinical trial management systems (CTMS), data analytics, and performance metrics (KPIs) to identify risks, spot trends, and make informed strategic decisions.
- Regulatory Strategy: A deep understanding of the regulatory environment and the ability to work with regulatory affairs to design operationally feasible trials that will meet FDA/EMA muster.
- Soft Skills: Do not underestimate these. At the Director level, leadership, influence, cross-functional collaboration, and executive communication are paramount. Your ability to inspire a team and present confidently to the C-suite is just as important as your technical knowledge.
---
Job Outlook and Career Growth

The future for Directors of Clinical Operations is