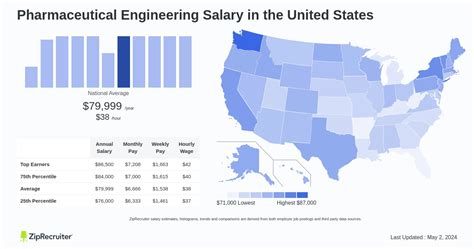
Welcome. If you're here, it’s likely because you're driven by a dual passion: a fascination with complex scientific processes and a desire to make a tangible impact on human health. You're considering a career that sits at the very heart of modern medicine—a field where a single process improvement can lead to the production of millions of life-saving doses. That field is pharmaceutical engineering, and it's not only one of the most impactful careers you can choose, but also one of the most financially rewarding.
The path of a pharmaceutical engineer is demanding. It requires a rigorous mind, an unwavering attention to detail, and a deep sense of responsibility. But the rewards are commensurate with the challenges. We're not just talking about a job; we're talking about a career with a national average salary well into the six figures, offering immense stability and growth potential. Many senior-level engineers and managers comfortably earn over $150,000, with top-tier experts in high-demand areas exceeding $200,000 per year.
I once had the opportunity to tour a biologics manufacturing facility where they were producing a breakthrough cancer therapy. Watching the lead process engineer explain the intricate network of bioreactors, chromatography skids, and fill-finish lines, I didn't just see stainless steel and technology. I saw the physical embodiment of hope, meticulously engineered to be safe, effective, and scalable. That engineer's work was the final, critical bridge between a brilliant scientific discovery in a lab and a patient in a hospital bed. This is the profound reality of this profession, and understanding its financial landscape is the first step toward making it your own.
This guide is designed to be your definitive resource. We will dissect every component of a pharmaceutical engineer's compensation, explore the factors that can maximize your earning potential, and lay out a clear roadmap for you to follow.
### Table of Contents
- [What Does a Pharmaceutical Engineer Do?](#what-does-a-pharmaceutical-engineer-do)
- [Average Pharmaceutical Engineering Salary: A Deep Dive](#average-pharmaceutical-engineering-salary-a-deep-dive)
- [Key Factors That Influence Salary](#key-factors-that-influence-salary)
- [Job Outlook and Career Growth](#job-outlook-and-career-growth)
- [How to Get Started in This Career](#how-to-get-started-in-this-career)
- [Conclusion: Is a Career in Pharmaceutical Engineering Right for You?](#conclusion-is-a-career-in-pharmaceutical-engineering-right-for-you)
What Does a Pharmaceutical Engineer Do?

At its core, a pharmaceutical engineer is a specialized type of chemical or process engineer who bridges the gap between research and development (R&D) and full-scale manufacturing of pharmaceutical drugs, vaccines, and other biologics. While a research scientist might discover a molecule that can fight a disease in a test tube, it's the pharmaceutical engineer who figures out how to safely, consistently, and economically produce millions or even billions of doses of that molecule in a highly regulated environment.
Their work is a complex blend of engineering principles, chemistry, biology, and regulatory science. They design, implement, monitor, and optimize all the processes and equipment involved in drug production. This isn't just about mixing chemicals; it's about mastering incredibly sensitive processes under the strictest quality standards in the world, known as Current Good Manufacturing Practices (cGMP).
Core Responsibilities and Typical Projects:
- Process Design & Development: Taking a process from the lab bench scale (milliliters) and scaling it up to pilot plants (liters) and eventually to full commercial manufacturing (thousands of liters). This involves complex calculations, equipment selection, and process modeling.
- Equipment & Facility Design: Designing and specifying the equipment used in manufacturing, such as bioreactors, filtration systems, chromatography columns, and lyophilizers (freeze-dryers). They also contribute to the design of the manufacturing facility itself, including cleanrooms and critical utility systems like Water-for-Injection (WFI).
- Process Validation (IQ/OQ/PQ): This is a critical regulatory requirement. Engineers perform and document rigorous testing to prove that equipment and processes consistently produce the expected results.
- Installation Qualification (IQ): Verifying that equipment is installed correctly.
- Operational Qualification (OQ): Verifying that the equipment operates according to its specifications.
- Performance Qualification (PQ): Verifying that the process, using the installed and operational equipment, consistently produces a product that meets all quality attributes.
- Troubleshooting & Optimization: When a manufacturing run has a problem (e.g., lower-than-expected yield, an impurity), engineers are the detectives who investigate the root cause and implement corrective and preventive actions (CAPAs). They are constantly looking for ways to make processes more efficient, safer, and more robust.
- Regulatory Compliance & Documentation: The mantra of the pharmaceutical industry is: "If it wasn't documented, it didn't happen." Engineers spend a significant amount of time writing, reviewing, and approving documents like Standard Operating Procedures (SOPs), Batch Records, validation protocols, and technical reports to ensure compliance with FDA and other global regulatory agencies.
### A "Day in the Life" of a Mid-Career Process Engineer
To make this more concrete, let's imagine a day for "Alex," a Process Engineer with 5 years of experience at a large biologics company.
- 8:00 AM - 8:30 AM: Alex arrives and checks the overnight process data from the ongoing manufacturing campaign for a monoclonal antibody drug. They use a SCADA (Supervisory Control and Data Acquisition) system to review key parameters like temperature, pH, and dissolved oxygen in the bioreactors. Everything looks within spec.
- 8:30 AM - 9:30 AM: Daily operations meeting with the manufacturing, quality assurance (QA), and maintenance teams. They discuss the previous day's performance, any minor deviations, and the plan for the current shift. Alex provides an update on a new filter type they are planning to test next week.
- 9:30 AM - 12:00 PM: "Desk time." Alex works on a validation protocol for a new mixing tank that will be installed next month. This involves defining the test parameters, setting acceptance criteria, and detailing the exact steps for the OQ and PQ. It's meticulous work that requires deep process knowledge and an understanding of FDA guidelines.
- 12:00 PM - 1:00 PM: Lunch with colleagues, discussing a tricky chromatography purification issue.
- 1:00 PM - 3:00 PM: "On the floor." Alex puts on full gowning (booties, gown, hairnet, mask, gloves) to enter the manufacturing suite. They are overseeing a critical step in the downstream purification process, observing the operators and ensuring the SOP is being followed precisely. They answer a technical question from an operator about a pressure reading on a filtration skid.
- 3:00 PM - 4:30 PM: Alex leads a cross-functional team meeting to investigate a minor deviation from last week's batch. Using root cause analysis tools like a fishbone diagram, they identify a potential issue with a sensor calibration. Alex is tasked with writing the investigation report and defining the CAPA, which will involve updating the calibration SOP.
- 4:30 PM - 5:00 PM: Final email checks and planning for the next day. Alex responds to a query from the R&D team about the feasibility of scaling up a new cell line, providing input on the potential challenges at the manufacturing scale.
This blend of analytical desk work, hands-on floor support, and collaborative problem-solving is the essence of the pharmaceutical engineering role.
Average Pharmaceutical Engineering Salary: A Deep Dive
Now, let's get to the numbers. The financial compensation for a pharmaceutical engineering career is a significant draw, reflecting the high level of skill, responsibility, and education required. It's a field that invests heavily in its talent.
It's important to note that the U.S. Bureau of Labor Statistics (BLS) does not have a dedicated category for "Pharmaceutical Engineer." Instead, these professionals are typically classified under Chemical Engineers or, in some biologics-focused roles, Biomedical Engineers. The pharmaceutical industry is one of the highest-paying sectors for these disciplines.
According to the BLS, the median annual wage for Chemical Engineers was $112,960 in May 2023. The top 10 percent earned more than $178,940. For Biomedical Engineers, the median annual wage was $101,040, with the top 10 percent earning more than $158,580. Pharmaceutical engineers typically fall into the upper-middle to top-tier of these ranges.
Let's look at more specific, industry-focused data from reputable salary aggregators.
- Salary.com (as of late 2023/early 2024) reports the average Pharmaceutical Process Engineer salary in the United States is around $115,000, with a typical range falling between $105,000 and $127,000.
- Glassdoor (as of early 2024) estimates the total pay for a Pharmaceutical Engineer in the US is $126,000 per year, with an average salary of $101,000 and additional pay (bonuses, profit sharing) averaging $25,000.
- Payscale (as of early 2024) places the average base salary for a Process Engineer in the pharmaceutical manufacturing industry at approximately $94,000, with a total pay range from $70,000 to $136,000+ when including bonuses.
These figures provide a strong baseline, but the most useful way to understand your potential earnings is to break them down by experience level. The salary progression in this field is steep and rewarding for those who commit to growing their skills.
### Pharmaceutical Engineering Salary by Experience Level
Here is a consolidated and realistic breakdown of what you can expect to earn at different stages of your career. These figures represent base salary and do not include the significant bonuses and other compensation common in the industry.
| Experience Level | Typical Job Titles | Typical Base Salary Range (Annual) |
| :--- | :--- | :--- |
| Entry-Level (0-2 years) | Associate Engineer, Process Engineer I | $75,000 - $95,000 |
| Mid-Career (3-8 years) | Process Engineer II, Senior Engineer | $95,000 - $130,000 |
| Senior (9-15 years) | Senior Engineer, Principal Engineer, Engineering Manager | $130,000 - $175,000 |
| Lead / Expert (15+ years) | Principal/Staff Engineer, Director of Engineering | $175,000 - $220,000+ |
*Source: Aggregated data from Salary.com, Glassdoor, Payscale, and industry recruitment reports for 2023-2024.*
### Beyond the Base Salary: Understanding Total Compensation
A pharmaceutical engineer's salary is just one piece of the puzzle. Total compensation is a much more accurate reflection of your earnings, and in this industry, it can be substantial.
- Annual Bonuses: This is the most common form of additional compensation. Bonuses are almost universal in for-profit pharma companies and are typically tied to both individual and company performance.
- Mid-Level Engineers: Can expect bonuses in the range of 8% to 15% of their base salary.
- Senior/Managerial Roles: Can see bonuses from 15% to 25% or more.
- *Example:* A Senior Engineer earning a $140,000 base salary could receive a bonus of $14,000 to $21,000, bringing their total cash compensation to $154,000 - $161,000.
- Stock Options & Restricted Stock Units (RSUs): For publicly traded companies, especially large pharmaceutical corporations and high-growth biotech firms, equity is a major part of the compensation package.
- RSUs: A grant of company shares that vest over a period of time (typically 3-4 years). This is a powerful tool for wealth creation and long-term retention.
- Stock Options: Common in startups, giving employees the right to buy company stock at a predetermined price in the future. High risk, but potentially enormous reward if the company succeeds.
- Profit Sharing: Some companies distribute a portion of their annual profits to employees, often as a direct deposit or a contribution to their retirement accounts.
- Retirement Benefits (401k/403b): The industry standard is excellent. Many large pharma companies offer a generous 401k match, often matching 100% of your contribution up to 5-6% of your salary, and may even add an additional automatic contribution on top of that.
- Health and Wellness Benefits: Comprehensive health, dental, and vision insurance with low premiums and deductibles is the norm. Other common perks include generous paid time off (PTO), parental leave, tuition reimbursement for advanced degrees, and wellness stipends.
When you add these components together, the total compensation package for a pharmaceutical engineer is one of the most competitive across all engineering disciplines. An engineer with a base salary of $120,000 could easily have a total compensation value approaching $150,000 or more when bonuses, stock, and benefits are factored in.
Key Factors That Influence Your Pharmaceutical Engineering Salary
While the national averages provide a great starting point, your personal earning potential is not a single number. It's a dynamic figure influenced by a combination of your qualifications, choices, and market realities. Mastering these factors is the key to maximizing your compensation throughout your career. This is the most critical section for understanding how to actively increase your value.
### 1. Level of Education and Certifications
Your educational foundation is the price of entry, and advanced credentials can unlock higher starting salaries and more specialized, lucrative roles.
- Bachelor's Degree (B.S.): A Bachelor of Science in Chemical Engineering is the gold standard and the most common entry point into the profession. Other related degrees like Biomedical Engineering, Mechanical Engineering, or a B.S. in Pharmaceutical Sciences can also be gateways, but a ChemE degree provides the most direct and versatile path. A strong GPA from a reputable engineering program is crucial for landing top-tier entry-level positions.
- Master's Degree (M.S. or M.Eng.): Pursuing a Master's degree can provide a significant salary bump, often in the range of 10-15% for a starting role. It's particularly valuable if you want to specialize in a specific area like biochemical engineering, process controls, or pharmaceutical manufacturing. More importantly, it can qualify you for R&D-adjacent roles or make you a more competitive candidate for leadership tracks later in your career. Many companies offer tuition reimbursement, making it possible to earn a Master's degree while working.
- Doctorate (Ph.D.): A Ph.D. is typically reserved for those who want to work in pure research and development, lead early-stage process development teams, or serve as a subject matter expert (SME) in a highly technical area (e.g., cell therapy process science). The starting salary for a Ph.D. is substantially higher than for a B.S. graduate, often beginning at the mid-career level ($120,000+), but the career path is more specialized.
- Professional Certifications: Certifications demonstrate a commitment to your craft and specialized expertise.
- Professional Engineer (P.E.) License: While not always required in the pharmaceutical industry (unlike in civil or construction engineering), having a P.E. license can be a significant differentiator, especially for roles in facility design, utilities, or consulting. It can add a salary premium and is often a prerequisite for senior consulting roles.
- Lean Six Sigma (Green Belt, Black Belt): These certifications are *highly* valued. They prove your expertise in process optimization, statistical analysis, and waste reduction—skills that directly impact a company's bottom line. A Black Belt certification can make you a prime candidate for high-level process excellence or operational leadership roles and can command a salary premium of $10,000 or more.
- Project Management Professional (PMP): As engineers advance, they invariably take on more project management responsibilities. A PMP certification formalizes these skills and is extremely valuable for those on a management track, overseeing large capital projects or technology transfers.
### 2. Years of Experience
Experience is arguably the single most powerful driver of salary growth in this field. The industry places an immense premium on hands-on, practical knowledge gained in a cGMP environment. The salary curve is not linear; it accelerates significantly after the first few years as you move from executing tasks to leading them.
- Entry-Level (0-2 years): You're learning the ropes, absorbing knowledge about cGMP, and applying your academic training to real-world systems. Your focus is on execution, data collection, and supporting senior engineers. Salary Range: $75,000 - $95,000.
- Mid-Career (3-8 years): You are now a fully independent contributor. You own processes, lead small to medium-sized projects, troubleshoot complex problems, and begin to mentor junior engineers. This is where you see the most significant jump in both responsibility and salary. Salary Range: $95,000 - $130,000.
- Senior/Lead (9-15 years): You are now a technical authority. You're leading large, complex projects (like a new facility startup or a major technology transfer), setting technical direction, and influencing strategy. You might be a Principal Engineer (a top technical expert) or an Engineering Manager (leading a team). Your decisions have a major financial and operational impact. Salary Range: $130,000 - $175,000.
- Expert/Director (15+ years): At this level, you are a leader of leaders. As a Director or VP of Engineering/Manufacturing/Technical Operations, you are responsible for entire departments or sites. Your role is primarily strategic, focusing on budget, long-range planning, and corporate goals. Compensation at this level is heavily weighted towards bonuses and stock, with base salaries often exceeding $200,000 - $220,000.
### 3. Geographic Location
Where you work matters—a lot. Salaries for pharmaceutical engineers are not uniform across the country. They are heavily concentrated in and around major biopharma hubs, where a high density of companies competes for a limited pool of talent, driving up both wages and the cost of living.
Here's a look at some of the top-paying regions, with salary data often reflecting a 15-30% premium over the national average:
1. Boston/Cambridge, MA: Arguably the world's #1 biotech hub. The sheer concentration of Big Pharma, established biotechs, and innovative startups creates immense demand. Expect the highest salaries here, but also one of the highest costs of living.
2. San Francisco Bay Area, CA: Another global powerhouse, particularly strong in biotech and gene therapy. Similar to Boston, it offers top-tier salaries that are offset by an extremely high cost of living.
3. San Diego, CA: A major hub for life sciences, with a strong focus on diagnostics, genomics, and therapeutics. Offers high salaries with a slightly more manageable (though still high) cost of living than the Bay Area.
4. New Jersey / New York City Metro: The traditional "medicine chest" of the US, this area is home to the headquarters and major manufacturing sites of many pharmaceutical giants (e.g., Merck, Johnson & Johnson, Bristol Myers Squibb). It remains a top-paying region.
5. Raleigh-Durham (Research Triangle Park), NC: A rapidly growing hub that attracts major investment for its world-class research institutions and more affordable cost of living compared to the coastal hubs. Salaries are very competitive and go further here.
6. Philadelphia, PA / Delaware Valley: A burgeoning hub for cell and gene therapy, building on its historical pharma roots. Offers strong salaries and a more moderate cost of living.
Conversely, roles located in the Midwest or Southeast outside of these specific hubs, while still well-compensated, will likely be closer to the national median. A $120,000 role in Boston might be a $100,000 role in a lower-cost-of-living area.
### 4. Company Type & Size
The type of company you work for dramatically shapes your work environment, career trajectory, and compensation structure.
- Big Pharma (e.g., Pfizer, Eli Lilly, Merck): These global giants offer high base salaries, excellent benefits, and structured, predictable bonus plans. They provide incredible stability, extensive training resources, and opportunities to work on blockbuster drugs at a massive scale. The trade-off can be a more bureaucratic environment and potentially slower career progression.
- Biotech Startups (Pre-IPO or newly public): This is the high-risk, high-reward path. The base salary might be slightly lower than at a Big Pharma company. However, the key attraction is a significant grant of stock options or RSUs. If the company succeeds and goes public or is acquired, this equity can be life-changing. The work is fast-paced, you'll wear many hats, and you'll have a direct impact on the company's success.
- Contract Development & Manufacturing Organizations (CDMOs) (e.g., Lonza, Catalent): These companies are hired by other pharma/biotech firms to develop processes and manufacture their drugs. Working at a CDMO offers competitive salaries and unparalleled experience, as you'll be exposed to a wide variety of different products, technologies, and clients in a short period. It's an excellent way to accelerate your learning.
- Equipment Vendors & Consulting Firms: Companies that sell specialized equipment or engineering consulting services to the pharma industry also hire many pharmaceutical engineers. These roles often involve travel and a mix of technical sales, support, and design. Compensation can be very high, often with a commission or utilization-based bonus structure.
- Government (e.g., FDA, NIH): A role as a reviewer or compliance officer at the FDA offers a different path. While the top-end salary will be lower than in private industry, the work-life balance, job security, and federal benefits are exceptional. The experience gained is also highly valuable if you ever decide to transition back to the private sector.
### 5. Area of Specialization
Within pharmaceutical engineering, certain specializations are more in-demand and command higher salaries.
- Upstream vs. Downstream Processing (Biologics): In the world of biologics (drugs made from living cells), engineers specialize in either "Upstream" (growing the cells in bioreactors) or "Downstream" (purifying the target protein from the cell culture). Both are critical, but engineers with deep expertise in large-scale downstream purification (specifically with complex chromatography and filtration techniques) are often in very high demand.
- Cell & Gene Therapy: This is the cutting edge of medicine. The processes for manufacturing CAR-T cells or viral vectors for gene therapy are new, complex, and not yet fully standardized. Engineers with any experience in this space are among the most sought-after and highly compensated professionals in the entire industry.
- Automation & Process Control: Modern pharma manufacturing is highly automated. Engineers who can design, program, and manage systems like DeltaV or Rockwell are invaluable. This skill set blends traditional process engineering with software and control theory, making it a rare and lucrative combination.
- Process Validation & Quality Engineering: With the FDA's intense focus on data integrity and process robustness, engineers who specialize in validation and quality systems (e.g., managing CAPAs, performing risk assessments) are always in demand. These roles require a meticulous, detail-oriented mindset and a deep understanding of regulations.
### 6. In-Demand Skills
Beyond your title or specialization, specific, demonstrable skills on your resume can directly translate to a higher salary offer.
- Technical Skills:
- cGMP Expertise: This is non-negotiable. Deep, practical knowledge of 21 CFR Parts 210 and 211 is the foundation of your value.
- Process Modeling Software: Proficiency in tools like Aspen Suite or SuperPro Designer for process simulation and modeling.
- Statistical Analysis: Mastery of statistical software like JMP or Minitab for Design of Experiments (DoE) and statistical process control (SPC).
- Single-Use Technology (SUT): Experience with the design and implementation of disposable manufacturing systems (bags, tubing, filters) is increasingly critical, especially in biologics.
- Data Historians: Experience with data systems like PI Historian for process monitoring and analysis.
- Soft Skills:
- Project Management: The ability to lead a project from conception to completion, on time and on budget.
- Cross-Functional Communication: The ability to clearly explain complex technical issues to non-engineers (e.g., management, finance, regulatory affairs).
- Problem-Solving under Pressure: The resilience and analytical skill to troubleshoot a critical manufacturing deviation when a multi-million dollar batch is on the line.
- Regulatory Interaction: Experience writing sections of regulatory filings (like a BLA or NDA) or participating in an FDA inspection is a huge value-add.
Job Outlook and Career Growth

Choosing a career is an investment, and like any good investment, you want to see strong, long-term growth prospects. For pharmaceutical engineers, the future is exceptionally bright. The demand for these professionals is driven by powerful, sustained trends in global health, technology, and demographics.
### Strong Job Growth Projections
As mentioned, we can use the U.S. Bureau of Labor Statistics (BLS) data for Chemical and Biomedical Engineers as a strong proxy for the pharmaceutical engineering field.
- The BLS projects employment of Chemical Engineers to grow 8 percent from 2022 to 2032, which is much faster than the average for all occupations. The BLS explicitly states that demand will be strong in pharmaceutical manufacturing.
- Employment of Biomedical Engineers is projected to grow 5 percent over the same period, also faster than average, with growth fueled by an aging population and an increased focus on health solutions.
These statistics translate to thousands of new jobs each year. The underlying drivers of