Navigating the complex world of government regulation is crucial for companies in science and technology, and at the heart of this process is the Regulatory Affairs Specialist. This critical role offers not only a challenging and impactful career but also significant financial rewards. For those with a keen eye for detail and a passion for science and law, this profession provides a stable and lucrative path, with average salaries often ranging from $70,000 to over $130,000 per year.
This guide will break down the salary you can expect as a Regulatory Affairs Specialist, the key factors that drive your earning potential, and the promising future of this essential profession.
What Does a Regulatory Affairs Specialist Do?

A Regulatory Affairs (RA) Specialist acts as the crucial link between a company and regulatory authorities like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Their primary goal is to ensure that a company's products—be they pharmaceuticals, medical devices, or biologics—meet all legal requirements for safety and efficacy.
Think of them as strategic navigators and expert translators. They translate complex scientific data into compliant regulatory submissions and interpret dense government regulations into actionable strategies for the company. Key responsibilities include:
- Preparing, writing, and submitting applications and registrations for new products.
- Ensuring that product labeling, advertising, and manufacturing processes comply with all applicable regulations.
- Advising product development teams on regulatory requirements to avoid costly delays.
- Communicating with regulatory agencies to address questions and facilitate approvals.
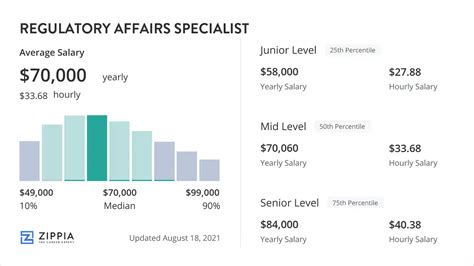
Average Regulatory Affairs Specialist Salary
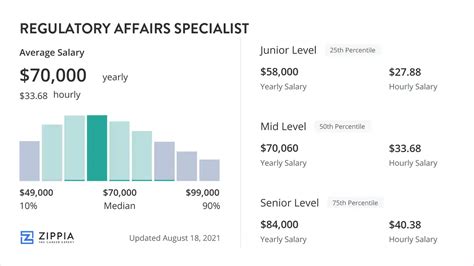
The compensation for a Regulatory Affairs Specialist is highly competitive, reflecting the specialized knowledge and critical importance of the role.
According to data compiled from leading salary aggregators, the average base salary for a Regulatory Affairs Specialist in the United States typically falls between $85,000 and $95,000 per year.
However, this is just an average. The complete salary range is broad and depends heavily on factors like experience and location:
- Payscale reports a typical range of $63,000 to $119,000 for base salary.
- Salary.com places the median salary slightly higher at $91,442, with a common range of $79,985 to $104,263.
- Glassdoor indicates an average base pay of around $90,500, with total pay (including bonuses and profit sharing) reaching closer to $98,000.
For professionals advancing in their careers, the titles and salaries grow. A Regulatory Affairs Manager can expect to earn a median salary of over $140,000, while a Director or Vice President of Regulatory Affairs can command a salary well into the $200,000s.
Key Factors That Influence Salary

Your specific salary as a Regulatory Affairs Specialist isn't a single number—it's a reflection of your unique skills, background, and work environment. Here are the most significant factors that influence your earning potential.
### Level of Education
While a bachelor's degree in a life science (like biology, chemistry, or pharmacy) is typically the minimum requirement, advanced education significantly boosts earning power.
- Bachelor's Degree: This is the entry point for most specialist roles.
- Master's Degree: A Master of Science in Regulatory Affairs, Regulatory Science, or a related field can increase starting salaries and accelerate career advancement. It demonstrates a specialized commitment and deeper understanding of the field.
- Doctoral Degree (Ph.D., Pharm.D., M.D.): Professionals with a doctorate command the highest salaries. Their advanced scientific expertise is invaluable for complex product submissions, particularly in cutting-edge areas like biologics and gene therapy. Furthermore, holding a professional certification, such as the Regulatory Affairs Certification (RAC) from the Regulatory Affairs Professionals Society (RAPS), can further enhance salary and job prospects.
### Years of Experience
Experience is arguably the most critical factor in determining salary. The regulatory field values a proven track record of successful submissions and interactions with agencies.
- Entry-Level (0-2 years): Professionals starting their careers can expect a salary in the range of $65,000 to $80,000. They typically handle more routine tasks and support senior team members.
- Mid-Career (3-9 years): With several years of experience, specialists gain more autonomy and manage more complex projects. Their salaries typically rise to $85,000 to $115,000.
- Senior-Level (10+ years): A senior specialist or manager with a decade or more of experience develops deep strategic insight. They lead teams, shape regulatory strategy, and command salaries of $120,000+.
### Geographic Location
Where you work matters. Salaries for regulatory professionals are highest in major pharmaceutical and biotechnology hubs, which also have a higher cost of living. According to RAPS salary survey data, top-paying metropolitan areas in the U.S. include:
- San Francisco Bay Area, CA
- Boston/Cambridge, MA
- San Diego, CA
- Washington D.C. / Maryland
- Raleigh-Durham (Research Triangle Park), NC
Salaries in these regions can be 15-30% higher than the national average to compensate for the competitive job market and living expenses.
### Company Type
The size and type of your employer play a significant role in your compensation package.
- Large Pharmaceutical/Biotech Companies: These corporations (e.g., Pfizer, Johnson & Johnson, Amgen) generally offer the highest base salaries, robust benefits packages, and structured bonus programs.
- Small to Mid-Sized Companies & Startups: While base salaries may be slightly lower, these companies often offer stock options or other equity incentives, which can have a significant upside if the company is successful.
- Contract Research Organizations (CROs): CROs manage regulatory and clinical trial work for multiple clients. Salaries here are competitive and offer exposure to a wide variety of products and therapeutic areas.
- Consulting Firms: Experienced specialists who become consultants can earn very high incomes, though this path often requires a strong personal brand and extensive network.
### Area of Specialization
The type of product you regulate directly impacts your value. More complex and novel products require deeper expertise and, therefore, command higher pay.
- Biologics and Gene Therapies: This is often the highest-paying area due to the complexity of the science and the evolving regulatory landscape.
- Pharmaceuticals: A traditional and stable area with strong salary potential.
- Medical Devices: This field is incredibly diverse, from simple instruments to complex software as a medical device (SaMD) and combination products. Specialists with expertise in high-risk devices or digital health often earn more.
- Food, Cosmetics, or Veterinary Medicine: These sectors also require regulatory oversight but generally offer salaries that are more modest compared to the medical and biotech industries.
Job Outlook

The future for Regulatory Affairs Specialists is exceptionally bright. As scientific innovation accelerates and global markets become more interconnected, the need for skilled professionals to manage the regulatory process is greater than ever.
While the U.S. Bureau of Labor Statistics (BLS) doesn't have a dedicated category for Regulatory Affairs Specialists, the closely related field of Compliance Officers provides a strong proxy. The BLS projects that employment for Compliance Officers will grow by 4 percent from 2022 to 2032. However, for specialized roles in pharmaceuticals and medical devices, industry experts anticipate much faster growth due to:
- An aging population driving demand for new medical treatments.
- The rise of personalized medicine, biologics, and digital health technologies.
- Increasingly stringent and harmonized global regulations.
This consistent demand creates strong job security and upward mobility for those in the field.
Conclusion

A career as a Regulatory Affairs Specialist is an outstanding choice for individuals who blend a scientific mind with a meticulous and strategic approach. It offers a direct way to contribute to public health by ensuring that safe and effective products reach the market.
Key Takeaways:
- Strong Earning Potential: With a national average salary approaching six figures and significant room for growth, this is a financially rewarding career.
- Experience is King: Your salary will grow substantially as you gain experience and a track record of success.
- Education and Specialization Pay Off: Advanced degrees and expertise in high-demand areas like biologics or medical device software will maximize your income.
- A Secure and Growing Field: The critical need for regulatory oversight ensures strong job security and a positive career outlook for years to come.
For those considering this path, investing in a strong scientific education and actively seeking opportunities to learn about regulatory strategy will pave the way for a successful and prosperous career.