Introduction

Do you possess a unique blend of scientific curiosity and a love for the written word? Have you ever considered a career where you could bridge the gap between complex medical data and the people who need to understand it—be they doctors, patients, or regulators? If so, the field of medical writing might be your calling. It's a profession that is not only intellectually stimulating and impactful but also surprisingly lucrative, with the average salary of a medical writer often reaching well into the six-figure range for experienced professionals.
This isn't just a job; it's a vital role at the heart of healthcare innovation. Medical writers are the unsung heroes who translate dense clinical trial results, intricate pharmacological mechanisms, and complex regulatory requirements into clear, concise, and accurate documents. I once had the privilege of working alongside a team preparing a submission for a breakthrough oncology drug. Seeing the senior medical writer transform mountains of raw data into a compelling narrative that ultimately helped get a life-saving treatment to patients was a profound lesson in the power of this profession. They weren't just writing; they were building the very foundation of trust and understanding upon which modern medicine operates.
This guide is designed to be your definitive resource for understanding the financial landscape of a medical writing career. We will dissect every factor that influences your earning potential, from education and experience to geographic location and specialization. Whether you are a recent science graduate, a healthcare professional considering a career change, or an experienced writer looking for a new niche, this article will provide you with the data-driven insights and actionable steps you need to navigate your path to a successful and well-compensated career as a medical writer.
### Table of Contents
- [What Does a Medical Writer Do?](#what-does-a-medical-writer-do)
- [Average Salary of a Medical Writer: A Deep Dive](#average-salary-of-a-medical-writer-a-deep-dive)
- [Key Factors That Influence Salary](#key-factors-that-influence-salary)
- [Job Outlook and Career Growth](#job-outlook-and-career-growth)
- [How to Get Started in This Career](#how-to-get-started-in-this-career)
- [Conclusion](#conclusion)
What Does a Medical Writer Do?

At its core, a medical writer is a professional communicator who specializes in creating scientific and medical documentation. They act as a critical link between scientists, clinicians, pharmaceutical companies, regulatory bodies, and the public. Their primary responsibility is to take complex, technical information and present it in a clear, accurate, and audience-appropriate format. This requires a unique combination of scientific knowledge, analytical skills, and writing prowess.
The scope of their work is vast and varies significantly depending on their specialization and employer. A medical writer isn't just one type of job; it's a multifaceted career with several distinct paths.
Common types of documents and projects include:
- Regulatory Writing: This is one of the most technical and highly regulated areas. Writers create documents for submission to regulatory agencies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Examples include:
- Clinical Study Reports (CSRs)
- Investigator's Brochures (IBs)
- Common Technical Document (CTD) summaries
- New Drug Application (NDA) or Biologics License Application (BLA) components
- Protocols for clinical trials
- Medical Communications (MedComms): This field focuses on educating healthcare professionals (HCPs). It often involves a blend of science and marketing. Documents include:
- Manuscripts for publication in peer-reviewed medical journals
- Abstracts and posters for scientific conferences
- Slide decks for presentations and advisory boards
- Continuing Medical Education (CME) materials
- Product monographs and sales training materials
- Patient and Consumer Health Writing: This involves creating easy-to-understand content for a non-scientific audience. Clarity and empathy are paramount. Examples are:
- Patient education brochures and websites
- Informed Consent Forms (ICFs)
- Health and wellness articles for magazines or blogs
- Content for pharmaceutical or hospital websites
- Medical Marketing and Advertising: This involves creating promotional materials for drugs, devices, or healthcare services, while adhering to strict advertising regulations. This can include website copy, email campaigns, and detail aids for sales representatives.
### A Day in the Life of a Medical Writer
To make this role more tangible, let's imagine a day for "Dr. Elena Vance," a Senior Medical Writer at a mid-sized pharmaceutical company, specializing in regulatory writing.
- 8:30 AM: Elena starts her day by checking emails. She sees a query from a statistician regarding data tables for a Clinical Study Report (CSR) she's drafting for a Phase 3 trial. She also has a meeting reminder for the project team call at 10:00 AM.
- 9:00 AM: She opens the CSR document. Using a company-provided template and style guide, she begins writing the efficacy results section, meticulously cross-referencing the statistical analysis plan and the data tables provided by the biostatistics team.
- 10:00 AM: Elena joins a cross-functional team call with the clinical project manager, the lead statistician, and a medical director. They discuss the timeline for the upcoming regulatory submission. Elena provides an update on the CSR progress and raises a point about an inconsistency she found between two data sets, which the team agrees to investigate.
- 11:00 AM: Back to writing. She incorporates feedback from the medical director on a previous draft of the safety section, ensuring every statement is supported by data and written with absolute precision.
- 1:00 PM: Lunch break, where she might read a new article in a relevant medical journal to stay current.
- 2:00 PM: Elena switches tasks to a different project. She's been asked to review a draft of a manuscript for a medical journal that another writer prepared. She checks for scientific accuracy, clarity, adherence to journal guidelines (e.g., ICMJE), and consistency with the company's publication strategy.
- 4:00 PM: She spends the last hour of her day working on a less intensive task: preparing a simple protocol amendment for a small, ongoing study. This involves updating a few sections and routing the document through the company's electronic document management system (like Veeva Vault) for review and approval.
- 5:00 PM: Elena logs off, having contributed to multiple critical stages of the drug development lifecycle through her precise and disciplined communication skills.
This example highlights the blend of independent writing, collaborative teamwork, and detail-oriented analysis that defines the medical writing profession.
Average Salary of a Medical Writer: A Deep Dive

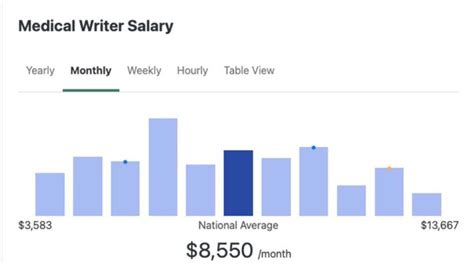
Now, let's get to the core of the matter: compensation. The salary of a medical writer is highly competitive and reflects the specialized skills and knowledge required for the role. It's a field where your expertise is directly rewarded in your paycheck.
While the U.S. Bureau of Labor Statistics (BLS) doesn't have a dedicated category for "Medical Writer," it groups them under "Technical Writers." As of May 2023, the BLS reports a median annual wage for Technical Writers of $83,730. The lowest 10 percent earned less than $53,040, and the highest 10 percent earned more than $138,060. However, it's widely recognized within the industry that medical writing, being a highly specialized subset, commands a significantly higher salary than the general technical writing average.
More specific salary aggregators provide a clearer picture. Let's examine the data from several reputable sources, as of late 2023 and early 2024:
- Salary.com: Reports the average Medical Writer salary in the United States is $93,506, with a typical range falling between $83,235 and $105,379. This platform's data is often based on HR-reported compensation information, giving it strong credibility.
- Glassdoor: Shows a total pay estimate for a Medical Writer in the US at $119,776 per year, with an estimated base salary of $100,569 and additional pay (bonuses, profit sharing) of around $19,207. This data is user-submitted and reflects real-world compensation packages.
- Payscale: Places the average base salary for a Medical Writer at $84,606 per year. Their data also indicates a wide range, from $61,000 for entry-level positions to over $124,000 for highly experienced writers.
The consensus from these sources is clear: a six-figure salary is not an anomaly but a realistic and common benchmark for mid-career and senior medical writers.
### Salary by Experience Level
Your earnings will grow substantially as you gain experience, master complex document types, and prove your value. Here is a typical progression, synthesized from the sources above:
| Experience Level | Typical Title(s) | Average Base Salary Range (USA) | Key Responsibilities & Expectations |
| :--- | :--- | :--- | :--- |
| Entry-Level
(0-2 years) | Medical Writer I, Associate Medical Writer | $65,000 - $85,000 | Learning industry standards, supporting senior writers, working on less complex documents (e.g., protocol amendments, simple reports, slide decks). Heavy focus on training and development. |
| Mid-Career
(3-7 years) | Medical Writer II, Medical Writer III, Senior Medical Writer | $90,000 - $125,000 | Independently managing and writing complex documents (e.g., CSRs, manuscripts), leading document review cycles, beginning to mentor junior writers, interacting with cross-functional teams. |
| Senior / Principal
(8+ years) | Senior/Principal Medical Writer, Manager of Medical Writing, Medical Writing Lead | $125,000 - $160,000+ | Leading writing strategy for entire clinical programs, managing teams of writers, providing expert consultation to senior management, handling the most complex and high-stakes submissions. |
| Freelance / Contractor | Freelance Medical Writer | Varies Greatly (often $90 - $175+ per hour) | Experts who work on a project basis. Hourly rates are high to compensate for lack of benefits, taxes, and inconsistent work. Highly experienced freelancers can earn well over $200,000 annually. |
*Note: These are national averages. Your specific salary will be heavily influenced by the factors discussed in the next section.*
### Beyond the Base Salary: Understanding Total Compensation
Your paycheck is only part of the story. The total compensation package for a medical writer, especially one working for a pharmaceutical company or a large Contract Research Organization (CRO), is often very attractive.
- Annual Bonuses: Performance-based bonuses are common and can range from 5% to 20% (or more for senior leadership) of your base salary. They are often tied to company performance and individual achievements, such as meeting submission deadlines.
- Stock Options/Restricted Stock Units (RSUs): Particularly in publicly traded pharmaceutical and biotech companies, equity is a significant part of compensation. This can add tens of thousands of dollars to your annual earnings over the vesting period.
- Profit Sharing: Some companies offer a profit-sharing plan, distributing a portion of the company's profits among employees.
- Sign-On Bonuses: To attract top talent, especially for senior or specialized roles, companies frequently offer sign-on bonuses, which can range from $5,000 to $25,000 or more.
- Comprehensive Benefits: Standard benefits include excellent health, dental, and vision insurance; generous 401(k) or 403(b) matching programs; and ample paid time off (PTO).
- Professional Development: Many employers will pay for membership in professional organizations like the American Medical Writers Association (AMWA) and cover costs for attending conferences or pursuing relevant certifications, which is a valuable indirect financial benefit.
When evaluating a job offer, it's crucial to look beyond the base salary and consider the full value of the compensation package.
Key Factors That Influence Your Medical Writer Salary
While the national averages provide a good baseline, your individual salary as a medical writer will be determined by a combination of powerful factors. Understanding these levers is key to maximizing your earning potential throughout your career. This is the most critical section for anyone looking to strategically build their career and command a top-tier salary.
### ### Level of Education
Your educational background forms the foundation of your credibility and knowledge base. While a bachelor's degree in a life science is the minimum entry point, advanced degrees are highly valued and often directly correlate with higher starting salaries and faster career progression.
- Bachelor's Degree (BS/BA): A degree in biology, chemistry, biochemistry, pharmacy, or a related field is typically required. Individuals with just a bachelor's degree can certainly succeed but may start at the lower end of the salary spectrum and often need to supplement their education with certifications or a very strong portfolio.
- Master's Degree (MS): A Master's in a scientific discipline, public health (MPH), or a specialized writing program can provide a competitive edge and may lead to a starting salary that is $5,000 to $10,000 higher than a candidate with only a bachelor's degree.
- Advanced Terminal Degrees (PhD, PharmD, MD): This is the gold standard, particularly for roles in top-tier pharmaceutical companies, regulatory writing, and roles that require deep scientific expertise (e.g., writing for oncology or immunology). Professionals with a doctorate-level degree can often command starting salaries in the $100,000+ range. They are seen as subject matter experts (SMEs) and can more quickly advance to principal writer or strategic leadership roles. The ability to critically analyze and interpret complex data, a skill honed during doctoral training, is immensely valuable.
Certifications: While not always required, professional certifications can increase your marketability and salary.
- AMWA Certification: The American Medical Writers Association offers an extensive certificate program. Earning an AMWA certificate demonstrates a commitment to the profession and a mastery of core competencies.
- Board of Editors in the Life Sciences (BELS) Certification: Earning the credential of Editor in the Life Sciences (ELS) is a prestigious mark of editorial skill and is highly respected, particularly for writers involved in publications and manuscript editing.
### ### Years and Quality of Experience
As the salary table above shows, experience is arguably the single most important factor. However, it's not just about the number of years but the *quality* and *type* of experience.
- 0-2 Years (The Learning Phase): Your primary goal is to learn. You are paid to absorb information about drug development, regulatory processes (like ICH guidelines), and company-specific procedures. Your salary is an investment the company is making in you.
- 3-7 Years (The Execution Phase): You are now a reliable, independent contributor. You can be trusted to lead the writing of a major document like a CSR from start to finish. You have successfully navigated review cycles, managed timelines, and worked effectively with cross-functional teams. Your salary increases significantly as you transition from a net "learner" to a net "producer."
- 8+ Years (The Strategy Phase): You are now a strategic asset. You are not just writing documents; you are contributing to submission strategy, mentoring teams, and improving processes. Your experience allows you to foresee potential issues and proactively solve them. At this level, you can transition into management or become a Principal Writer, an individual contributor role with a salary rivaling that of a manager. Writers with deep experience in a high-demand therapeutic area (e.g., oncology, rare diseases) are especially valuable.
### ### Geographic Location
Where you live and work has a massive impact on your paycheck, largely due to variations in the cost of living and the concentration of life sciences companies. The rise of remote work has somewhat flattened these differences, but geographic hotspots still command a premium.
| Location Category | Example Cities/Regions | Typical Salary Impact | Why the Difference? |
| :--- | :--- | :--- | :--- |
| Top-Tier BioPharma Hubs | Boston/Cambridge, MA; San Francisco Bay Area, CA; San Diego, CA | +15% to +30% above national average | High concentration of major pharma, biotech, and medical device companies. Intense competition for talent drives salaries up. Very high cost of living. |
| Major Life Science Hubs | Raleigh-Durham (Research Triangle Park), NC; Philadelphia, PA; New Jersey; Seattle, WA | +5% to +15% above national average | Strong presence of CROs, pharmaceutical companies, and academic medical centers. High demand for writers with a more moderate cost of living than top-tier hubs. |
| Other Major Metropolitan Areas | Chicago, IL; Austin, TX; Atlanta, GA | At or slightly above national average | Growing biotech scenes and a presence of large companies. Salaries are competitive but don't have the same premium as the established hubs. |
| Lower Cost-of-Living / Remote | Most other regions / Fully remote positions | -5% to -15% below hub averages | Fewer local opportunities may depress wages. For remote roles, companies may adjust salary based on the employee's location, though some have a single national pay scale. |
*Source: Analysis based on salary data from Glassdoor and Salary.com, which allow for location-based filtering.* A medical writer in Boston might earn $140,000 for a role that pays $115,000 in a lower-cost area.
### ### Company Type & Size
The type of organization you work for is a major determinant of your salary and overall compensation package.
- Large Pharmaceutical Companies ("Big Pharma"): (e.g., Pfizer, Johnson & Johnson, Merck) These companies typically offer the highest base salaries and most robust benefits packages. They have deep pockets, highly structured career ladders, and offer significant bonuses and stock options. The work is often highly specialized and process-driven.
- Contract Research Organizations (CROs): (e.g., IQVIA, ICON, PPD) CROs are the workhorses of the industry, managing clinical trials and writing services for multiple pharma clients. Salaries are very competitive, often rivaling Big Pharma, but the pace can be more demanding as you may work on projects for different clients with different standards simultaneously. It's an excellent place to gain a wide breadth of experience quickly.
- Biotech and Small-to-Mid-Sized Pharma: These companies can be more variable. A well-funded, late-stage biotech might offer salaries competitive with Big Pharma, often with a more significant equity component (stock options) to compensate for higher risk. Early-stage startups may offer lower base salaries but potentially lucrative stock options if the company is successful.
- Medical Device Companies: Salaries are generally competitive and on par with pharmaceutical companies, though the regulatory pathways and document types (e.g., Clinical Evaluation Reports - CERs) are different.
- Medical Communications Agencies: These agencies serve pharma and device clients, focusing on publications, slide decks, and marketing materials. Salaries can be strong, but the work is often very fast-paced and client-driven. Bonus structures are common and tied to agency performance.
- Hospitals and Academic Medical Centers: These roles often focus on grant writing, manuscript preparation for faculty, or patient education. Salaries are typically lower than in the corporate sector, but the work-life balance may be better, and the benefits (like tuition remission) can be excellent.
### ### Area of Specialization
Within medical writing, some niches are more lucrative than others. This is a classic case of supply and demand.
- Regulatory Writing: This is consistently the highest-paid specialization. The work is incredibly high-stakes—a poorly written document can delay the approval of a multi-billion dollar drug. The knowledge required (FDA/EMA guidelines, eCTD structure) is highly specific and not easily acquired. Writers specializing in regulatory submissions, especially for complex biologics or cell and gene therapies, are in constant demand.
- Medical Communications (Publications): This is also a very well-compensated field. Writing manuscripts for high-impact journals requires a deep understanding of the data and the ability to craft a compelling scientific story. It is a highly respected and stable career path.
- Medical Marketing / Promotional Writing: Salaries can be very high, but this field requires a different skillset—a blend of scientific accuracy and persuasive, creative communication. Success in this area can lead to high-paying roles in brand management or marketing strategy.
- Patient Education and Health Literacy: While incredibly important and rewarding, these roles may have a slightly lower salary ceiling compared to regulatory writing. They are more common in non-profit organizations or hospital settings, which typically have lower pay scales than for-profit corporations.
- Continuing Medical Education (CME): This is a stable niche focused on creating accredited educational materials for physicians. Salaries are solid and fall within the general mid-to-upper range for medical writers.
### ### In-Demand Skills
Beyond your core qualifications, certain specific skills can significantly boost your value and, therefore, your salary.
- Therapeutic Area Expertise: Having deep experience in a "hot" and complex area like Oncology, Immunology, Rare Diseases, or Gene Therapy makes you a highly sought-after candidate.
- Knowledge of Regulatory Guidelines: Fluency in the language of the FDA, EMA, and ICH (International Council for Harmonisation) is non-negotiable for regulatory writers and a huge plus for all others.
- Statistical Literacy: You don't need to be a statistician, but you must be able to understand statistical concepts, read analysis plans, and intelligently discuss data with biostatisticians.
- Project Management: The ability to manage timelines, coordinate reviews, and lead meetings is crucial as you move into senior roles.
- Proficiency with Industry Software: Experience with electronic document management systems (EDMS) like Veeva Vault, citation management software like EndNote, and collaborative platforms is expected.
By strategically developing these factors, you can actively steer your career towards higher levels of compensation.
Job Outlook and Career Growth

The future for medical writers is exceptionally bright. The demand for skilled professionals who can clearly and accurately communicate complex medical information is not just stable—it's growing.
The U.S. Bureau of Labor Statistics projects that employment for Technical Writers (the category including medical writers) will grow 7 percent from 2022 to 2032, which is faster than the average for all occupations. The BLS explicitly states, "Growth will be driven by the continuing expansion of scientific and technical products... Demand for technical writers is expected to be strong in the life sciences."
For medical writers specifically, the growth drivers are even more potent:
1. Explosion in R&D: The global pharmaceutical and biotechnology industries are continuously investing billions in research and development. Every new drug, biologic, and medical device requires an enormous volume of written documentation for its development, approval, and marketing.
2. Increasingly Complex Science: The rise of personalized medicine, cell and gene therapies, and complex biologics means that the underlying science is more complicated than ever. This increases the need for specialists who can understand and explain it.
3. Aging Global Population: An aging population leads to a higher prevalence of chronic diseases, driving more research and a greater need for both professional- and patient-facing medical information.
4. Globalization of Clinical Trials: As clinical trials become more global, there is a greater need for writers who can prepare documentation that meets the requirements of multiple regulatory bodies (e.g., FDA, EMA, PMDA in Japan).
5. Focus on Health Literacy: There is a growing recognition of the importance of clear communication with patients. This drives demand for writers who can create accessible and understandable health content, from clinical trial materials to public health websites.
### Emerging Trends and Future Challenges
To stay ahead, medical writers must be aware of the evolving landscape:
- Artificial Intelligence (AI): AI tools are emerging that can assist with tasks like generating initial drafts, checking for consistency, and formatting. Rather than a threat, savvy medical writers will see this as an opportunity. The future role will involve using AI as a powerful assistant, focusing human expertise on strategy, critical analysis, and nuanced storytelling—tasks AI cannot replicate. Those who master these tools will be more efficient and valuable.
- Remote Work and a Global Talent Pool: The pandemic solidified remote work as a viable and often preferred model in medical writing. This gives writers more flexibility and access to jobs outside their geographic area. However, it also means companies can hire from a global talent pool, increasing competition.
- Data Visualization: The ability to work with graphic designers or use tools to create clear, compelling charts, graphs, and infographics is becoming an increasingly important skill. A picture can often tell a more powerful story than a paragraph of text.
### How to Advance and Stay Relevant
Advancement in medical writing isn't always a linear path up a corporate ladder. There are multiple routes to growth and higher earnings:
1. Become a Deeper Specialist: Double down on a high-demand therapeutic area (like oncology) or a complex document type (like regulatory submission modules). Become the go-to expert.
2. Transition to Management: For those with leadership skills, moving into a "Manager of Medical Writing" role is a natural progression. This involves hiring, training, and managing a team of writers.
3. Move into an Adjacent Role: The skills of a medical writer are highly transferable. Experienced writers can move into roles in Clinical Development, Regulatory Affairs Strategy, Medical Science Liaison (MSL), or Publication Planning.
4. Embrace Freelancing: Once you have significant experience (typically 7-10+ years) and a strong network, freelance medical writing offers the ultimate flexibility and can be extremely lucrative. Top-tier freelance regulatory writers can command rates of $150/hour or more.
5. Continuous Learning: The science never stops advancing, and neither should your education. Stay current by reading journals, attending webinars and conferences (like the AMWA Annual Conference), and pursuing new certifications.
How to Get Started in This Career

Breaking into the field of medical writing can seem daunting, but with a strategic approach, it is absolutely achievable. Here is a step-by-step guide for aspiring medical writers.
### Step 1: Build the Right Educational Foundation
As discussed, a scientific background is essential.
- Obtain a degree in a life science (biology, chemistry, pharmacy, nursing) or a related field. If your degree is in a non-science field like English or journalism, you must supplement it with science coursework (e.g., anatomy, physiology, pharmacology) to demonstrate scientific literacy.
- **