In the heart of every medical breakthrough, every life-saving drug, and every innovative treatment protocol lies a foundation of meticulously managed, rigorously analyzed data. This is the world of the Clinical Data Analyst (CDA), a critical role that bridges the gap between raw clinical trial information and the actionable insights that drive modern medicine forward. If you possess a keen analytical mind, a passion for detail, and a desire to contribute to human health, this career path offers not just profound personal satisfaction but also significant financial rewards.
A career as a Clinical Data Analyst is one of the most stable and increasingly lucrative positions in the healthcare and life sciences industries. With the explosion of data generated from clinical trials, electronic health records (EHRs), and wearable technology, the demand for professionals who can manage, interpret, and protect this information has never been higher. This demand is directly reflected in the compelling salary packages offered to qualified candidates. Across the United States, a Clinical Data Analyst can expect an average salary that often falls between $75,000 and $105,000, with senior professionals and specialists in high-demand areas earning well over $130,000 annually.
Early in my career consulting for a burgeoning biotech firm, I witnessed a pivotal moment where the entire trajectory of a promising Phase II trial hinged on the work of a single, sharp-eyed clinical data analyst. She identified a subtle but persistent anomaly in data collection from one of the trial sites—an anomaly that, left unchecked, would have invalidated months of work and millions of dollars in investment. Her diligence not only saved the trial but underscored for me the immense value and responsibility vested in this role; they are the guardians of integrity in the pursuit of medical progress.
This comprehensive guide is designed to be your definitive resource for understanding the financial landscape of a clinical data analyst career. We will dissect salary expectations, explore the key factors that drive compensation, and provide a clear, step-by-step roadmap to help you launch or advance your own journey in this exciting field.
### Table of Contents
- [What Does a Clinical Data Analyst Do?](#what-does-a-clinical-data-analyst-do)
- [Average Clinical Data Analyst Salary: A Deep Dive](#average-clinical-data-analyst-salary-a-deep-dive)
- [Key Factors That Influence Salary](#key-factors-that-influence-salary)
- [Job Outlook and Career Growth](#job-outlook-and-career-growth)
- [How to Get Started in This Career](#how-to-get-started-in-this-career)
- [Conclusion: Is This the Right Career for You?](#conclusion-is-this-the-right-career-for-you)
What Does a Clinical Data Analyst Do?

A Clinical Data Analyst is far more than a number cruncher; they are a vital steward of clinical trial data, ensuring its accuracy, integrity, and readiness for statistical analysis and regulatory submission. They operate at the critical intersection of clinical research, data management, and information technology. Their primary objective is to guarantee that the data collected during a clinical study is clean, reliable, and compliant with stringent industry standards and government regulations, such as Good Clinical Practice (GCP) and FDA guidelines.
The role begins long before the first patient is enrolled in a trial. A CDA is often involved in the design and testing of the Case Report Forms (CRFs)—the tools used to collect patient data—and the setup of the Electronic Data Capture (EDC) systems that house this information. Once a trial is underway, they become the frontline defenders of data quality.
Core responsibilities and daily tasks typically include:
- Data Validation and Cleaning: The heart of the role. CDAs write and run validation checks (edit checks) within the clinical database to identify discrepancies, missing data, or illogical entries. For example, a check might flag a record where a male patient is listed as pregnant or where a lab value is outside a biologically plausible range.
- Query Management: When a data discrepancy is found, the CDA issues a "query" to the clinical site (the hospital or clinic conducting the trial). They manage the entire query lifecycle, from issuance to resolution, working closely with Clinical Research Associates (CRAs) to ensure timely and accurate responses from investigators.
- Database Management: They are responsible for maintaining the clinical trial database, including tasks like user access management, performing data updates, and preparing the database for "locking" at the end of a study. A database lock is a critical milestone, after which no further changes can be made to the data.
- Coding: CDAs often perform medical coding, translating verbatim terms for medical histories, adverse events, and medications into standardized dictionary terms using systems like MedDRA (Medical Dictionary for Regulatory Activities) and WHODrug (WHO Drug Dictionary). This ensures consistency in data reporting.
- Reporting and Reconciliation: They generate reports on data status, query metrics, and patient enrollment for the wider clinical team. They also perform serious adverse event (SAE) reconciliation, ensuring that data in the clinical database matches the data in the separate safety database.
- Documentation: Meticulous documentation is paramount. CDAs contribute to and maintain key study documents like the Data Management Plan (DMP), which outlines all data handling procedures for a specific trial.
> ### A Day in the Life of a Clinical Data Analyst
>
> 8:30 AM - 9:30 AM: Morning Review & Status Check
> Arrive, grab coffee, and log into the EDC system (e.g., Medidata Rave, Oracle Clinical). Review overnight data entry from clinical sites across different time zones. Run a quick dashboard report to check the status of outstanding data queries and see if any urgent safety alerts have been triggered.
>
> 9:30 AM - 12:00 PM: Data Validation & Query Issuance
> This is core focus time. Run pre-programmed validation scripts (often using SAS or SQL) against new data. The scripts flag dozens of potential issues: a patient's visit date is out of sequence, a lab result is missing, a required field was left blank. For each valid issue, you draft a clear, concise query in the EDC system and route it to the appropriate clinical site for clarification.
>
> 12:00 PM - 1:00 PM: Lunch
>
> 1:00 PM - 2:30 PM: Cross-Functional Team Meeting
> Join the weekly study team meeting with the Clinical Research Manager, CRAs, and a Biostatistician. You present your data status report: "We have 95% of data entered for Cycle 3, with an average query resolution time of 4.5 days. Site 102 has a high number of outstanding queries, and I've flagged this for the assigned CRA to follow up on." The Biostatistician asks for a preliminary data cut for an interim analysis, and you discuss the timeline for cleaning the necessary datasets.
>
> 2:30 PM - 4:00 PM: SAE Reconciliation & Medical Coding
> Perform the critical task of Serious Adverse Event (SAE) reconciliation. You pull a report from the clinical database and the separate safety database, comparing them line-by-line to ensure every SAE is consistently recorded in both systems. Afterwards, you tackle the medical coding queue, standardizing reported adverse events like "stomach ache" to the precise MedDRA term "Abdominal pain."
>
> 4:00 PM - 5:00 PM: Documentation & Planning
> Update the Data Management Plan (DMP) to reflect a minor protocol amendment approved last week. You also spend time reviewing the specifications for a new report requested by the project manager. Before logging off, you prioritize your tasks for tomorrow, noting that a large data import from a central lab is scheduled and will require immediate validation.
Average Clinical Data Analyst Salary: A Deep Dive
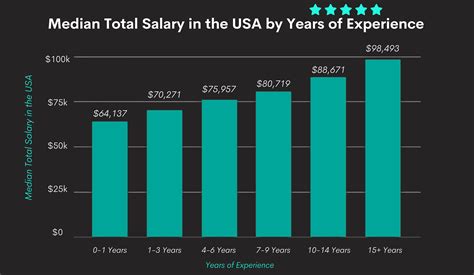
The salary of a Clinical Data Analyst is a compelling aspect of the career, reflecting the specialized skills and critical importance of the role. Compensation is not just a single number but a package that includes a competitive base salary, potential bonuses, and a robust benefits package. The figures below are synthesized from leading salary aggregators and industry data to provide a comprehensive and reliable picture of earning potential in the United States.
According to recent data, the salary landscape for Clinical Data Analysts is strong and varies based on several key factors, which we will explore in the next section.
- Salary.com reports that the median salary for a Clinical Data Analyst in the United States is approximately $91,660 as of late 2023, with the typical range falling between $79,880 and $104,260.
- Payscale provides a slightly broader range, indicating an average salary of around $73,500, but shows a total pay spectrum (including bonuses and profit sharing) from $55,000 to $101,000. This lower average may include more entry-level or academic roles.
- Glassdoor lists a national average salary of $99,500, with a likely range between $78,000 and $127,000, reflecting data from a large pool of user-submitted salary reports, often from corporate environments like pharmaceutical and biotech companies.
Taking a composite view of these authoritative sources, a reasonable expectation for a mid-career Clinical Data Analyst is a base salary in the $85,000 to $105,000 range. However, this is just the midpoint. Your individual earning potential can be significantly higher or lower depending on your experience, location, and skillset.
### Salary by Experience Level
Career progression brings a clear and significant increase in earning potential. Companies place a high premium on experience, as seasoned analysts can manage more complex trials, work more autonomously, and mentor junior staff.
| Experience Level | Typical Years of Experience | Average Annual Salary Range | Key Responsibilities & Expectations |
| :--- | :--- | :--- | :--- |
| Entry-Level Clinical Data Analyst | 0 - 2 Years | $65,000 - $80,000 | Focus on learning study protocols, running standard edit checks, issuing basic queries, and performing data entry review under close supervision. |
| Mid-Career Clinical Data Analyst | 3 - 7 Years | $80,000 - $110,000 | Manages data for multiple studies or a complex, high-enrollment study. Designs and tests edit checks, writes sections of the Data Management Plan, and leads data reconciliation efforts. May begin to mentor junior analysts. |
| Senior/Lead Clinical Data Analyst | 8+ Years | $110,000 - $140,000+ | Serves as the lead data manager for large-scale, global clinical programs. Oversees all data management activities from startup to database lock, develops departmental standards (SOPs), liaises with vendors, and provides strategic input to the clinical team. Often responsible for mentoring the entire data management team for a project. |
*Source: Synthesized from Salary.com, Glassdoor, and Payscale data, 2023.*
### Beyond the Base Salary: Understanding Your Total Compensation
A Clinical Data Analyst's salary is more than just a paycheck. The total compensation package is a critical part of your financial picture and can add substantial value. When evaluating a job offer, be sure to consider:
- Annual Bonuses: Highly common in the pharmaceutical, biotech, and CRO industries. These are typically performance-based and can range from 5% to 15% of your base salary, often tied to both individual and company performance (e.g., meeting project timelines, successful database locks, or drug approvals).
- Profit Sharing & Stock Options: While more common in publicly traded biotech and large pharmaceutical companies, these can be a significant long-term wealth-building tool. Startups may offer more substantial equity grants in lieu of a higher base salary.
- Retirement Savings Plans: A 401(k) or 403(b) plan is standard. The key differentiator is the employer match. A competitive company will match your contributions up to a certain percentage (e.g., 100% match on the first 4-6% of your salary), which is essentially free money.
- Health and Wellness Benefits: Comprehensive health, dental, and vision insurance is a given. Look for companies that offer low-premium, low-deductible plans. Many also provide wellness stipends for gym memberships, mental health resources, and generous paid time off (PTO) policies.
- Professional Development: A great employer will invest in your growth. This can include reimbursement for professional certifications (like the CHDA), tuition assistance for a master's degree, or a budget for attending industry conferences like the SCDM (Society for Clinical Data Management) annual conference. This is a benefit that directly increases your future earning potential.
Key Factors That Influence Salary

While the national averages provide a useful benchmark, your specific salary as a Clinical Data Analyst will be determined by a dynamic interplay of several key factors. Understanding these variables is crucial for negotiating job offers, planning your career trajectory, and maximizing your lifetime earning potential. This section provides an exhaustive breakdown of the elements that have the most significant impact on your paycheck.
### ### Level of Education
Your educational background is the foundation upon which your career is built. While a bachelor's degree is typically the minimum requirement, advanced degrees and specialized training can unlock higher starting salaries and more senior roles.
- Bachelor's Degree: This is the most common entry point. A Bachelor of Science (B.S.) is often preferred over a Bachelor of Arts (B.A.). Degrees in Life Sciences (Biology, Chemistry), Public Health, Health Informatics, Statistics, or a related field are most desirable. Graduates with a relevant bachelor's degree can expect to enter at the lower end of the salary spectrum, typically in the $65,000 to $75,000 range.
- Master's Degree: Pursuing a master's degree can provide a significant salary advantage, often allowing you to bypass more junior-level roles and start at a mid-level salary. A Master of Science (M.S.) or Master of Public Health (MPH) in Biostatistics, Epidemiology, Clinical Research, or Health Informatics is highly valued. A candidate with a relevant master's degree might command a starting salary $10,000 to $20,000 higher than a candidate with only a bachelor's degree.
- Doctorate (Ph.D.): While a Ph.D. is not necessary for a CDA role, it is common for those in adjacent, higher-paying roles like Biostatistician or Data Scientist within a clinical setting. A Ph.D. might be overqualified for a standard CDA I or II position but would be a strong candidate for a leadership role in data science or biometrics, commanding salaries well into the $150,000+ range.
- Professional Certifications: Certifications act as a powerful signal of expertise and commitment to the field. They can directly influence salary negotiations.
- Certified Clinical Data Manager (CCDM®) from the Society for Clinical Data Management (SCDM): This is the gold standard for experienced data managers and can lead to senior and lead roles.
- Certified Health Data Analyst (CHDA®) from AHIMA: This certification demonstrates proficiency in data analysis, management, and governance within the broader healthcare context and is highly respected.
- SAS Certified Base Programmer/Advanced Programmer: As SAS remains a dominant tool in the industry, official certification can provide a distinct advantage and a potential salary bump, particularly within CROs and large pharma companies.
### ### Years of Experience
Experience is arguably the single most powerful driver of salary growth in this field. The clinical research industry is risk-averse; it values professionals with a proven track record of successfully managing complex data in a highly regulated environment.
- 0-2 Years (Entry-Level): At this stage, you are learning the fundamentals. Your salary ($65k - $80k) reflects that you are in a training and development phase, working under close supervision. Your value is in your potential and your ability to learn quickly.
- 3-7 Years (Mid-Career): You have managed at least one or two studies from start to finish. You can work independently, troubleshoot complex data issues, and understand the nuances of different therapeutic areas. Your salary climbs steeply into the $80k - $110k range as you become a reliable, core member of the study team. You have moved from a cost center (trainee) to a value-generating asset.
- 8-15+ Years (Senior/Lead/Principal): You are now a subject matter expert. You are likely leading data management for entire clinical programs, not just single studies. You mentor junior staff, develop standards, manage vendors, and provide strategic direction. Your deep knowledge of regulations, various EDC systems, and complex trial designs (e.g., adaptive trials) makes you highly valuable. This is where salaries push into the $110k - $140k range, with principal-level and associate director roles exceeding $150,000.
### ### Geographic Location
Where you work has a dramatic impact on your salary. This is largely driven by the local cost of living and the concentration of pharmaceutical, biotech, and medical device companies in the region. Major life sciences hubs command the highest salaries but also have the highest living expenses.
Top-Paying Metropolitan Areas and States:
- San Francisco Bay Area, CA: The epicenter of biotech innovation. Salaries here are consistently the highest in the nation to compensate for the extreme cost of living. A mid-career CDA could earn $120,000 - $150,000+.
- Boston/Cambridge, MA: Another primary hub with a dense concentration of pharma giants, biotech startups, and world-class research hospitals. Salaries are very competitive, often 15-25% above the national average.
- San Diego, CA: A major life sciences cluster with a strong focus on genomics and biotechnology. Salaries are high, rivaling those in Boston and the Bay Area.
- New York, NY / Northern New Jersey: This region is home to many of the world's largest pharmaceutical companies (e.g., Pfizer, Merck, Johnson & Johnson). The proximity to Wall Street and a high cost of living drive salaries upward.
- Raleigh-Durham (Research Triangle Park), NC: A fast-growing hub for CROs and R&D facilities. While the cost of living is more moderate, the demand for talent keeps salaries highly competitive, often 5-15% above the national average.
States with Lower-than-Average Salaries:
Salaries tend to be lower in regions with a lower cost of living and fewer life sciences headquarters. This includes many states in the Midwest and the South (outside of major hubs like RTP). However, the rise of remote work is beginning to level the playing field, allowing analysts in lower-cost areas to earn higher salaries by working for companies based in major hubs.
### ### Company Type & Size
The type of organization you work for is a major determinant of your salary, work culture, and career path.
- Large Pharmaceutical Companies ("Big Pharma"): (e.g., Pfizer, Roche, Novartis)
- Salary: Generally offer the highest base salaries, excellent bonuses, and top-tier benefits.
- Culture: Highly structured, process-driven, and often bureaucratic. Offers stability and clear career ladders but may be slower-paced.
- Biotechnology Companies: (Can range from small startups to large, established firms like Amgen or Gilead)
- Salary: Established biotech firms offer pay competitive with Big Pharma. Early-stage startups may offer lower base salaries but compensate with potentially lucrative stock options.
- Culture: Can be more innovative, fast-paced, and less siloed. You may have the opportunity to wear many hats. Job security can be lower at smaller, pre-revenue companies.
- Contract Research Organizations (CROs): (e.g., IQVIA, Labcorp, PPD)
- Salary: Salaries are competitive but can sometimes be slightly lower than at a pharma/biotech sponsor. However, the experience gained is immense.
- Culture: Very fast-paced, high-volume environment. You will work on studies for many different sponsors across various therapeutic areas. This is an excellent place to build a diverse skillset quickly, making you highly marketable later on.
- Academic Research Centers and Hospitals:
- Salary: Typically pay the least, often 15-30% below corporate roles.
- Culture: Mission-driven, focused on research and patient care. Often offers excellent work-life balance, generous vacation policies, and strong tuition benefits, which can be a valuable trade-off.
### ### Area of Specialization
Within the clinical data analysis field, certain specializations can increase your value and earning potential. This isn't about specializing in "IT vs. Marketing," but rather in specific, high-value domains within clinical research.
- Therapeutic Area: Expertise in a complex and high-investment area like Oncology is extremely valuable. Cancer trials often have complex designs, massive data volumes, and unique endpoints (e.g., RECIST criteria). Analysts with deep oncology experience are in high demand and can command premium salaries. Other valuable areas include Immunology, Rare Diseases, and Central Nervous System (CNS) disorders.
- Technical Specialization: Becoming a power user or subject matter expert in a specific platform can be lucrative. This could be a leading EDC system like Medidata Rave, a data visualization tool like Tableau or Spotfire, or having advanced programming skills for data integration and transformation.
- Standards Expertise: Deep knowledge of CDISC standards (SDTM, ADaM) is a major differentiator. These are the standards required by the FDA for data submission. An analyst who can not only manage data but also ensure it is structured for seamless submission is a huge asset, particularly in later-stage trials.
- Real-World Evidence (RWE): This emerging field analyzes data from EHRs, insurance claims, and patient registries outside of traditional clinical trials. Analysts with skills in handling this "messy" real-world data are at the forefront of a major industry trend and are highly sought after.
### ### In-Demand Skills
Beyond your degree and years of experience, a specific set of technical and soft skills will directly impact your salary. The more of these you master, the more leverage you have in negotiations.
High-Impact Technical Skills:
- SQL (Structured Query Language): The ability to directly query databases is a fundamental, non-negotiable skill for any serious data professional. It allows you to perform complex data investigations that go beyond the standard reports in an EDC system.
- SAS: For decades, SAS has been the statistical programming language of choice
