Introduction

Are you driven by a desire to be at the forefront of medical innovation? Do you possess a unique blend of scientific acumen, meticulous organization, and leadership prowess? If you're seeking a career that not only offers substantial financial rewards but also places you at the very heart of developing life-changing therapies, then the role of a Clinical Research Manager (CRM) might be your calling. This position is far more than a job; it is a critical command post in the complex, high-stakes world of clinical trials, where every decision can impact the future of healthcare.
The financial compensation for this level of responsibility is significant, with the average clinical research manager salary comfortably sitting in the six-figure range, often supplemented by generous bonuses and benefits. Top professionals in high-demand areas can see their total compensation soar well above $200,000 annually. I remember a mentor of mine, a seasoned CRM overseeing a pivotal oncology trial, once telling me, "We aren't just managing timelines and budgets. We are the guardians of the scientific process and the patient's hope. Every data point we verify is a step closer to an answer for a family waiting for a breakthrough." That sentiment perfectly captures the profound importance and rewarding nature of this career.
This comprehensive guide will serve as your definitive resource, exploring every facet of the Clinical Research Manager role. We will dissect salary expectations, analyze the factors that drive compensation, map out the career trajectory, and provide a step-by-step roadmap to help you launch or advance your journey in this dynamic and vital field.
### Table of Contents
- [What Does a Clinical Research Manager Do?](#what-does-a-clinical-research-manager-do)
- [Average Clinical Research Manager Salary: A Deep Dive](#average-clinical-research-manager-salary-a-deep-dive)
- [Key Factors That Influence Salary](#key-factors-that-influence-salary)
- [Job Outlook and Career Growth](#job-outlook-and-career-growth)
- [How to Get Started in This Career](#how-to-get-started-in-this-career)
- [Conclusion](#conclusion)
What Does a Clinical Research Manager Do?

A Clinical Research Manager is the operational linchpin of a clinical trial. If a clinical trial is an orchestra, the CRM is the conductor, ensuring every section—from regulatory compliance and patient recruitment to data collection and site management—plays in perfect harmony to produce a clear, reliable result. They are senior-level professionals responsible for the planning, execution, and oversight of one or more clinical studies, ensuring they are completed on time, within budget, and in strict adherence to regulatory protocols and Good Clinical Practice (GCP) guidelines.
Their role is a multifaceted blend of science, project management, finance, and human resources. They bridge the gap between the high-level strategy set by the study's sponsor (like a pharmaceutical company or research institution) and the day-to-day work performed by Clinical Research Associates (CRAs), Clinical Research Coordinators (CRCs), and investigative site staff.
Core Responsibilities and Daily Tasks:
A CRM's duties are extensive and vary based on the organization, but they generally encompass the following:
- Protocol Development and Oversight: Collaborating with medical experts and biostatisticians to develop the study protocol—the detailed "recipe" for the trial. They ensure the protocol is feasible, ethical, and designed to meet its scientific objectives.
- Team Leadership and Management: Hiring, training, and managing a team of clinical research professionals (such as CRAs). They set performance goals, conduct reviews, and provide mentorship to ensure the team is effective and motivated.
- Budget and Financial Management: Developing and managing the multi-million-dollar budgets associated with clinical trials. This includes forecasting costs, negotiating contracts with vendors and clinical sites, and tracking expenses to prevent overruns.
- Regulatory Compliance: Serving as the expert on regulations from bodies like the Food and Drug Administration (FDA) in the U.S. and the European Medicines Agency (EMA) in Europe. They ensure all study activities, documentation, and reporting are compliant to avoid costly delays or disqualification of data.
- Site Management and Monitoring: Overseeing the selection, initiation, and monitoring of clinical trial sites (hospitals, clinics). They ensure these sites have the proper resources, are recruiting eligible patients, and are collecting data accurately.
- Vendor Management: Selecting and managing third-party vendors, such as central laboratories, data management organizations, and interactive response technology (IRT) providers.
- Risk Management: Proactively identifying potential risks to the study's timeline, budget, or data integrity and developing mitigation plans.
- Reporting and Communication: Acting as the primary point of communication between the sponsor, the clinical sites, and the internal research team. They provide regular status updates and prepare comprehensive reports for senior management and regulatory bodies.
### A Day in the Life of a Clinical Research Manager
To make this tangible, let's imagine a day for "Dr. Anya Sharma," a CRM at a mid-sized biotech company overseeing a Phase II trial for a new Alzheimer's drug.
- 8:30 AM - Morning Huddle: Anya leads a video call with her team of five Clinical Research Associates (CRAs). They review key performance indicators (KPIs): patient enrollment rates across 20 sites, data query resolution times, and upcoming site monitoring visits. One CRA flags a site in Texas that is struggling with patient recruitment, and Anya tasks them with setting up a call with the site's Principal Investigator to troubleshoot.
- 10:00 AM - Budget Review: Anya meets with the finance department to review the quarterly trial budget. She justifies a recent cost increase due to the need for more expensive patient screening scans but demonstrates how she has offset this by renegotiating a contract with their data management vendor.
- 11:30 AM - Regulatory Communication: She drafts a formal response to an inquiry from the FDA regarding a minor protocol amendment. Her response must be precise, thorough, and scientifically sound.
- 1:00 PM - Lunch & Learn: Anya hosts a virtual lunch session for her team on new updates to the Good Clinical Practice (GCP) guidelines, ensuring everyone is up-to-date on the latest compliance standards.
- 2:00 PM - Steering Committee Meeting: She presents a progress report to the study's steering committee, which includes the Chief Medical Officer and other senior executives. She confidently presents the data, highlights achievements, transparently addresses the recruitment challenge, and outlines her plan to get it back on track.
- 4:00 PM - Problem Solving: A CRA calls in a panic: a freezer at a major clinical site has failed, potentially compromising hundreds of patient biological samples. Anya calmly walks the CRA through the site's contingency plan, directs them to quarantine the samples, and initiates the formal investigation and reporting process.
- 5:30 PM - Planning: Before logging off, she reviews the schedule for the next day, prioritizing a check-in with the struggling Texas site and preparing materials for an upcoming site initiation visit in California.
This example illustrates the CRM's dynamic role, requiring a constant switch between strategic oversight, hands-on problem-solving, and expert communication.
Average Clinical Research Manager Salary: A Deep Dive
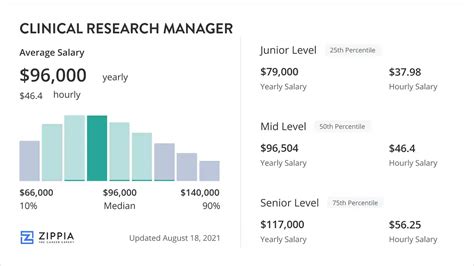
The compensation for a Clinical Research Manager reflects the high level of responsibility, specialized knowledge, and significant impact they have on a company's success. The salary is not a single number but a wide spectrum influenced by a multitude of factors we will explore in the next section. However, by synthesizing data from leading compensation and labor authorities, we can establish a clear picture of the earning potential.
According to the U.S. Bureau of Labor Statistics (BLS), Clinical Research Managers fall under the broader category of "Medical and Health Services Managers." This group earned a median annual wage of $110,680 as of May 2023. The lowest 10 percent earned less than $67,960, while the highest 10 percent earned more than $216,750. (Source: BLS, Occupational Outlook Handbook, 2024).
However, the BLS category is broad. For a more precise figure specific to the clinical research industry, we turn to specialized salary aggregators.
- Salary.com reports that the median annual salary for a Clinical Research Manager in the United States is approximately $135,468 as of May 2024. The typical salary range falls between $118,054 and $155,716. This range represents the middle 50% of earners, with significant potential for earnings on either side of this bell curve.
- Glassdoor provides a similar perspective, reporting an average base pay of $128,450 per year as of early 2024. Crucially, Glassdoor also estimates "Total Pay" (which includes bonuses, profit sharing, and other cash compensation) to be around $146,800 per year, highlighting that non-salary compensation forms a significant part of the overall package.
- Payscale data indicates an average base salary of approximately $115,000 per year, but it strongly emphasizes the dramatic salary growth with experience, with late-career CRMs earning substantially more.
In summary, a reasonable expectation for a mid-career Clinical Research Manager in the U.S. is a base salary in the $120,000 to $140,000 range, with total compensation, including bonuses, reaching $140,000 to $165,000 or higher.
### Salary Progression by Experience Level
Salary growth in this field is steep and directly correlated with experience. As a CRM gains expertise in managing more complex trials, navigating regulatory hurdles, and leading larger teams, their value—and compensation—skyrockets.
Here is a typical salary progression, combining data from the sources above:
| Experience Level | Years of Experience | Typical Base Salary Range | Typical Total Compensation Range (with Bonus) |
| :--- | :--- | :--- | :--- |
| Entry-Level CRM | 1-3 years | $95,000 - $115,000 | $105,000 - $125,000 |
| Mid-Career CRM | 4-9 years | $115,000 - $145,000 | $130,000 - $170,000 |
| Senior CRM | 10-15 years | $145,000 - $175,000 | $165,000 - $210,000 |
| Lead / Director-Level | 15+ years | $175,000+ | $210,000+ |
*Note: These are national averages. Geographic location and other factors discussed below can shift these ranges significantly.*
An "Entry-Level" CRM typically has prior experience as a senior CRA or a Clinical Trial Lead before moving into their first full management role. A "Senior CRM" often manages multiple complex, global trials or a specific portfolio of studies within a therapeutic area. A "Lead" or "Director" may have several CRMs reporting to them and hold strategic responsibility for an entire clinical development program.
### A Closer Look at Compensation Components
The headline salary is only part of the story. A comprehensive compensation package for a CRM is typically structured as follows:
- Base Salary: The fixed, annual salary paid out in regular paychecks. This is the foundation of the compensation package and is determined by the core factors of experience, location, and company type.
- Annual Performance Bonus: This is a highly significant component, especially in for-profit settings like pharmaceutical companies and Contract Research Organizations (CROs). Bonuses are typically tied to individual performance (e.g., meeting trial enrollment and timeline goals) and company performance. A typical target bonus can range from 10% to 25% of the base salary. For a CRM earning $140,000, this could mean an additional $14,000 to $35,000 per year.
- Stock Options / Restricted Stock Units (RSUs): In publicly traded biotech and pharmaceutical companies, equity is a common and lucrative incentive. RSUs or stock options give the manager ownership in the company, aligning their interests with long-term success. This can add tens of thousands of dollars to their annual compensation, particularly if the company's stock performs well.
- Sign-On Bonus: In a competitive hiring market, companies often offer a one-time sign-on bonus to attract top talent. This can range from a few thousand dollars to $20,000 or more for highly sought-after candidates.
- Comprehensive Benefits Package: This non-cash compensation is extremely valuable. It almost always includes:
- Health Insurance: Medical, dental, and vision plans, often with low premiums for employees and their families.
- Retirement Savings: A 401(k) or 403(b) plan, typically with a generous company match (e.g., matching 50-100% of employee contributions up to 6% of their salary).
- Paid Time Off (PTO): Generous vacation, sick leave, and paid holidays.
- Professional Development: A budget for attending conferences, obtaining certifications, and pursuing further education.
When evaluating a job offer, it's crucial to look at the Total Compensation Package, not just the base salary. A lower base salary with an exceptional bonus structure, generous equity, and a strong 401(k) match can often be more financially advantageous in the long run.
Key Factors That Influence Salary

While we've established a baseline, the actual salary a Clinical Research Manager earns is a complex equation with many variables. Understanding these factors is crucial for negotiating job offers and strategically planning your career path to maximize your earning potential. This section provides a granular breakdown of the six primary drivers of a CRM's salary.
### 1. Level of Education
In a field grounded in science and medicine, education is the foundational element of a CRM's qualifications and directly impacts their starting salary and long-term trajectory.
- Bachelor's Degree: A bachelor's degree in a life science (e.g., Biology, Chemistry, Biochemistry, Nursing) is the standard minimum requirement to enter the clinical research field. Individuals can work their way up from a coordinator or CRA role to a manager position with a BS/BA, but they may face a salary ceiling compared to those with advanced degrees.
- Master's Degree: An advanced degree significantly enhances earning potential. A Master of Science (MS) in Clinical Research, a Master of Public Health (MPH), or a Master of Health Administration (MHA) are particularly valuable. These programs provide specialized knowledge in biostatistics, epidemiology, research ethics, and healthcare management, making candidates more competitive. A CRM with a master's degree can expect to earn 5-15% more than a counterpart with only a bachelor's degree and similar experience.
- Doctoral Degree (PhD, PharmD, MD): The highest earners in this field often hold a terminal degree. A PhD in a relevant scientific field, a Doctor of Pharmacy (PharmD), or a Medical Doctor (MD) degree positions a professional for the top-tier roles, especially in early-phase clinical development or highly complex therapeutic areas. These individuals not only manage trials but also contribute to scientific strategy. Their starting salaries as CRMs can be 15-30% higher, and they have a clearer path to senior leadership roles like Director of Clinical Operations or Chief Medical Officer.
- Professional Certifications: While not a formal degree, certifications act as a powerful salary booster. The most recognized certifications include:
- Project Management Professional (PMP): This certification from the Project Management Institute (PMI) is highly valued because clinical trial management is, at its core, a form of complex project management. It demonstrates expertise in budgeting, scheduling, and risk management, which can add a 5-10% premium to a salary.
- ACRP Certified Professional (ACRP-CP): Offered by the Association of Clinical Research Professionals (ACRP).
- Certified Clinical Research Coordinator (CCRC®) / Certified Clinical Research Associate (CCRA®): While these are for roles leading up to manager, holding them demonstrates a foundational mastery of GCP and trial conduct.
### 2. Years of Experience
Experience is arguably the single most powerful determinant of a CRM's salary. The clinical research industry places an enormous premium on professionals who have a proven track record of successfully navigating the complexities and unforeseen challenges of clinical trials.
- Early Career (1-3 years as CRM): A professional transitioning into a CRM role, typically after 5-7 years as a Senior CRA or Clinical Trial Lead, will be at the lower end of the salary spectrum ($95k-$115k). They are proving their ability to manage budgets, lead teams, and handle increased responsibility.
- Mid-Career (4-9 years as CRM): This is where salaries see the most significant growth. A mid-career CRM has managed multiple trials, likely in different phases (I, II, III) and therapeutic areas. They have faced and resolved major issues (e.g., regulatory audits, site non-compliance, safety events). Their expertise is reliable and commands a salary in the $115k-$145k range.
- Senior Career (10+ years as CRM): A senior CRM is a strategic leader. They may be managing global, multi-center, blockbuster trials worth hundreds of millions of dollars. They mentor junior managers and contribute to departmental strategy. Their deep institutional knowledge and industry network make them invaluable, pushing their base salaries well into the $145k-$175k+ range, with total compensation often exceeding $200k.
### 3. Geographic Location
Where you work matters—a lot. Salaries for Clinical Research Managers vary dramatically based on the concentration of pharmaceutical companies, biotech firms, and research institutions, as well as the regional cost of living.
Major biotech and pharmaceutical hubs offer the highest salaries to attract top talent in a competitive market.
High-Paying Metropolitan Areas (15-30% above national average):
- San Francisco Bay Area, CA: The epicenter of biotech innovation. Salaries here are consistently the highest in the nation to offset the extreme cost of living. A CRM here can easily command a base salary of $160,000 to $190,000.
- Boston/Cambridge, MA: Another top-tier hub with a dense concentration of Big Pharma, biotech startups, and world-class academic research centers. Salaries are comparable to the Bay Area.
- San Diego, CA: A major life sciences cluster with a strong focus on genomics and biotechnology.
- Raleigh-Durham (Research Triangle Park), NC: A key hub for CROs and pharmaceutical companies, offering high salaries with a more moderate cost of living compared to CA and MA.
- New York/New Jersey Corridor: The long-standing home of many pharmaceutical giants ("Big Pharma"), offering very competitive compensation packages.
Average-Paying Areas (At or near the national average):
- Major cities like Chicago, IL; Philadelphia, PA; and Austin, TX have growing life science sectors and offer salaries that align with the national average.
Lower-Paying Areas (Below national average):
- Salaries tend to be lower in regions with fewer research-intensive industries, such as the rural Midwest and parts of the Southeast. However, the lower cost of living can sometimes offset the smaller paycheck. The rise of remote work is beginning to flatten these differences slightly, but location-based pay adjustments are still standard practice.
### 4. Company Type & Size
The type and size of the employing organization have a profound impact on salary, work culture, and career opportunities.
- Large Pharmaceutical Companies ("Big Pharma"):
- Salary: Generally offer high base salaries, excellent benefits, and strong, structured bonus programs. They have the resources to pay top-of-market rates.
- Environment: Highly structured, process-driven, and often bureaucratic. Offers stability, clear career ladders, and the opportunity to work on large-scale, global trials for potential blockbuster drugs.
- Contract Research Organizations (CROs):
- Salary: Extremely competitive, often offering the highest base salaries and most aggressive bonus structures in the industry. Their business model is based on efficiency and expertise, so they pay a premium for top-performing managers.
- Environment: Fast-paced, high-pressure, and demanding. CRMs at CROs often work on projects for multiple sponsors and across various therapeutic areas, leading to rapid skill development. Examples include IQVIA, Labcorp, and PPD.
- Biotechnology Companies (Small-to-Mid-Sized):
- Salary: Base salaries may be slightly lower than Big Pharma or CROs, but this is often compensated with significant equity (stock options). If the company is successful (e.g., gets a drug approved or is acquired), this equity can be life-changing.
- Environment: Dynamic, innovative, and often less bureaucratic. CRMs may have a broader range of responsibilities and more direct impact on company strategy. Job security can be lower, as it's tied to funding rounds and clinical trial success.
- Academic Medical Centers / Hospitals:
- Salary: Typically the lowest among the different employer types. Base salaries may be 10-20% lower than in the for-profit industry.
- Environment: Mission-driven, focused on investigator-initiated research. The pace can be slower, and the benefits (like pension plans and tuition remission) are often excellent. It's a great place for those passionate about pure science and patient care.
- Government (e.g., NIH, FDA, VA):
- Salary: Governed by federal pay scales (GS levels). Salaries are moderate and public, but the benefits, job security, and work-life balance are unparalleled.
- Environment: Bureaucratic and process-heavy, focused on public health and regulatory oversight.
### 5. Area of Specialization (Therapeutic Area)
Within clinical research, specialization matters. Just as a surgeon specializing in neurosurgery earns more than a general surgeon, a CRM specializing in a complex, high-investment therapeutic area can command a higher salary.
High-Paying Specializations:
- Oncology: Cancer research is the largest and most well-funded area in clinical development. The trials are incredibly complex, often involving novel immunotherapies, cell therapies (CAR-T), and targeted agents. Expertise in oncology is in high demand and pays a significant premium.
- Neurology / Central Nervous System (CNS): Trials for diseases like Alzheimer's, Parkinson's, and Multiple Sclerosis are long, difficult, and require specialized knowledge of cognitive and functional endpoints.
- Rare Diseases / Orphan Drugs: These trials involve small patient populations, complex logistics, and often novel genetic-based therapies. Managers with experience here are highly sought after.
- Cell and Gene Therapy: This cutting-edge field involves highly complex manufacturing and logistics ("chain of custody") and is one of the highest-paying specializations today.
Standard-Paying Specializations:
- Cardiology
- Infectious Diseases / Vaccines
- Metabolic Diseases (e.g., Diabetes)
- Dermatology
Expertise in a high-demand area can add another 5-15% to a CRM's salary compared to more common therapeutic areas.
### 6. In-Demand Skills
Beyond formal qualifications, a specific set of hard and soft skills can make a candidate more valuable and directly influence their salary offer.
High-Value Hard Skills:
- Clinical Trial Management Systems (CTMS): Proficiency in leading CTMS platforms like Veeva Vault, Medidata Rave, or Oracle Clinical is essential.
- Electronic Data Capture (EDC) Systems: Deep understanding of how data is captured and managed electronically.
- Budgeting and Financial Forecasting: The ability to accurately build and manage a multi-million-dollar trial budget.
- Global Trial Management: Experience managing trials across multiple countries, with knowledge of different regulatory environments (e.g., EMA, PMDA in Japan).
- Vendor Negotiation: A proven ability to negotiate favorable contracts with CROs, labs, and other vendors.
Critical Soft Skills (The "Value Multipliers"):
- Leadership and Mentoring: The ability to inspire and develop a high-performing team is a key differentiator for senior roles.
- Strategic Thinking: Moving beyond day-to-day execution to anticipate long-term risks and opportunities for a clinical program.
- Exceptional Communication and Influence: The
