Are you a meticulous planner with a passion for science and a desire to make a tangible impact on global health? Do you thrive at the intersection of project management, scientific inquiry, and human connection? If so, a career as a Clinical Study Manager (CSM), also known as a Clinical Trial Manager (CTM), might be your calling. This pivotal role is not only intellectually stimulating and profoundly rewarding but also offers a highly competitive compensation package. The average clinical study manager salary in the United States often exceeds $135,000 per year, with senior professionals in high-demand areas commanding salaries well over $180,000.
Early in my career consulting for a biopharmaceutical firm, I had a conversation with a senior CSM who was overseeing a complex Phase III oncology trial. She wasn't a scientist in a lab or a physician at the bedside, yet her work was the critical link ensuring a potentially life-saving therapy could one day reach patients. She described her role as "conducting an orchestra where every instrument is in a different city, speaks a different language, and the sheet music is a 5,000-page protocol." Her dedication and the sheer complexity she managed with grace left a lasting impression, underscoring the immense value and expertise this career demands.
This guide is designed to be your definitive resource, whether you are a Clinical Research Associate (CRA) looking for the next step, a student contemplating a career in clinical research, or a professional seeking to understand your earning potential. We will dissect the clinical study manager salary from every angle, explore the factors that drive compensation, and provide a clear roadmap to launching and advancing in this dynamic field.
### Table of Contents
- [What Does a Clinical Study Manager Do?](#what-does-a-clinical-study-manager-do)
- [Average Clinical Study Manager Salary: A Deep Dive](#average-clinical-study-manager-salary-a-deep-dive)
- [Key Factors That Influence a Clinical Study Manager's Salary](#key-factors-that-influence-salary)
- [Job Outlook and Career Growth for Clinical Study Managers](#job-outlook-and-career-growth)
- [How to Become a Clinical Study Manager: A Step-by-Step Guide](#how-to-get-started-in-this-career)
- [Is a Career as a Clinical Study Manager Right for You?](#conclusion)
What Does a Clinical Study Manager Do?

A Clinical Study Manager is the operational linchpin of a clinical trial. While the Principal Investigator (PI) is responsible for the scientific integrity and medical oversight at a specific site, the CSM is responsible for the end-to-end operational management of the entire study, which may span dozens or even hundreds of sites across multiple countries. They are the ultimate project managers, tasked with ensuring a clinical trial is completed on time, within budget, and in strict compliance with regulatory standards (like FDA and ICH-GCP) and the study protocol.
Their responsibilities are vast and varied, blending strategic oversight with hands-on management. They don't just follow a plan; they create it, adapt it, and see it through to completion.
Core Responsibilities Include:
- Protocol Development and Feasibility: Collaborating with medical teams to develop a clinical trial protocol that is both scientifically sound and operationally feasible. They assess the likelihood of successfully recruiting patients and running the study within the proposed timelines and budget.
- Vendor and Site Management: Selecting, qualifying, and managing Clinical Research Organizations (CROs), central laboratories, and other third-party vendors. They are also heavily involved in identifying, selecting, and initiating clinical trial sites (hospitals, clinics).
- Budget and Timeline Management: Developing and meticulously tracking the study budget, which can run into the tens or hundreds of millions of dollars. They create and maintain detailed project timelines, identifying risks and developing contingency plans to mitigate delays.
- Team Leadership and Oversight: Leading the clinical operations team, which typically includes Clinical Research Associates (CRAs), Clinical Trial Assistants (CTAs), and other support staff. They ensure the team is properly trained, motivated, and performing effectively. While they may not directly manage all CRAs (who often report to a line manager), they provide study-specific direction and are accountable for the quality of monitoring.
- Regulatory Compliance and Quality Assurance: Ensuring that all aspects of the study adhere to Good Clinical Practice (GCP), Standard Operating Procedures (SOPs), and all applicable local and global regulations. They oversee the Trial Master File (TMF) and are often the primary point of contact during regulatory inspections or audits.
- Cross-Functional Collaboration: Serving as the central point of communication, liaising with numerous internal departments such as Regulatory Affairs, Data Management, Biostatistics, Medical Affairs, and Pharmacovigilance.
### A Day in the Life of a Clinical Study Manager
To make this role more tangible, let's walk through a hypothetical day:
- 8:30 AM - 9:30 AM: Start the day by reviewing the study dashboard. Check key performance indicators (KPIs): patient enrollment rates against targets, data query resolution times, and monitoring visit report submissions. Notice that enrollment is lagging in the EU region and make a note to address this.
- 9:30 AM - 11:00 AM: Lead a cross-functional study team meeting. The agenda includes an update from the lead CRA on a significant protocol deviation at a site in Texas, a presentation from Data Management on recent data trends, and a discussion with the supply chain team about an impending drug shortage. You facilitate the discussion, ensure action items are assigned, and make critical decisions to keep the study on track.
- 11:00 AM - 12:00 PM: Call with the Project Manager at the lead CRO. Discuss the lagging EU enrollment. Together, you brainstorm solutions: launching a targeted digital ad campaign for patient recruitment, conducting a motivational call with the site PIs, or potentially opening a new high-performing site in Germany. You agree on a set of actions and a follow-up date.
- 12:00 PM - 1:00 PM: Lunch while reviewing a draft of the revised Informed Consent Form that Regulatory Affairs just sent over.
- 1:00 PM - 2:30 PM: Financial deep dive. Meet with the clinical finance partner to review the monthly budget report. You analyze vendor invoices, forecast future expenses, and identify a potential budget over-run due to higher-than-expected screening failure rates, requiring a re-forecast.
- 2:30 PM - 4:00 PM: Co-monitoring visit preparation. You will be joining a junior CRA for a site visit next week to provide oversight and training. You review the site's performance history and the monitoring plan to prepare your agenda.
- 4:00 PM - 5:00 PM: Respond to a flurry of emails. One is from a PI with a question about the protocol's exclusion criteria. Another is from the head of clinical operations asking for a one-page summary of the study's status for an executive meeting.
- 5:00 PM - 5:30 PM: Update the study's risk management log with the issues identified today and the mitigation plans put in place. End the day by updating your to-do list for tomorrow.
This "day in the life" illustrates the dynamic, problem-solving nature of the job. A CSM must be able to switch contexts rapidly, from high-level strategy to minute operational details, all while leading a diverse, global team toward a common goal.
Average Clinical Study Manager Salary: A Deep Dive

The compensation for a Clinical Study Manager is a direct reflection of the immense responsibility and specialized skill set required for the role. It is a highly sought-after position, and the salary data confirms its value in the marketplace.
Let's break down the numbers from multiple authoritative sources to provide a clear and comprehensive picture of earning potential.
### National Averages and Salary Ranges
While figures vary slightly between data aggregators due to different methodologies and data sets, they paint a consistent picture of a lucrative career.
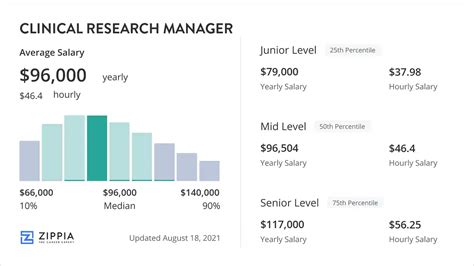
- Salary.com: As of late 2023 and early 2024, the median annual salary for a Clinical Trial Manager in the United States is $135,767. The typical salary range falls between $119,652 and $154,635. This represents the "middle 50%" of earners, with the top 10% earning over $170,188 per year.
- Glassdoor: This platform, which relies on user-submitted data, reports a total pay estimate of $148,500 per year for a Clinical Study Manager in the US, with an estimated base salary of around $129,000 and additional pay (bonuses, etc.) of nearly $20,000.
- Payscale: Payscale.com reports a slightly more conservative average base salary of $122,500 per year. However, it also highlights a wide range, with salaries stretching from $90,000 on the low end to $157,000 on the high end, before bonuses and profit sharing are considered.
Key Takeaway: A reasonable expectation for a mid-career Clinical Study Manager is a base salary in the $125,000 to $145,000 range, with total compensation often pushing into the $150,000+ territory.
### Salary by Experience Level
Experience is arguably the single most significant factor in determining a CSM's salary. The career path typically involves progressing from a more junior research role, and compensation rises steeply with demonstrated success in managing complex trials.
Here’s a breakdown of typical salary expectations by experience level, synthesized from industry data:
| Experience Level | Typical Years in Clinical Research | Typical Role Title(s) | Estimated Annual Base Salary Range |
| :--- | :--- | :--- | :--- |
| Entry-Level CSM | 3-5 years | Clinical Study Manager I, Associate CSM | $95,000 - $118,000 |
| Mid-Career CSM | 5-10 years | Clinical Study Manager II, Clinical Trial Manager | $118,000 - $145,000 |
| Senior CSM | 10+ years | Senior CSM, Principal CTM, Lead CTM | $145,000 - $175,000+ |
| Leadership / Director | 12+ years | Associate Director/Director of Clinical Ops | $180,000 - $250,000+ |
*Note: "Entry-Level CSM" does not mean entry-level to the workforce. It typically requires several years of prior clinical research experience, most commonly as a CRA.*
### Beyond the Base Salary: Understanding Total Compensation
A CSM's base salary is only one piece of the puzzle. The total compensation package is often substantially higher, especially when working for pharmaceutical sponsors or profitable biotech companies.
Common Components of a CSM's Compensation Package:
- Annual Bonus: This is a standard component of compensation in the pharmaceutical and biotech industries. It's typically performance-based, tied to both individual and company goals (e.g., meeting study enrollment timelines, successful database lock, positive company financial performance). A typical bonus target for a CSM ranges from 10% to 20% of their base salary. For a CSM earning $140,000, this could mean an additional $14,000 to $28,000 per year.
- Long-Term Incentives (LTIs) / Equity: This is particularly common in publicly traded pharmaceutical companies and biotech firms. LTIs can come in the form of:
- Restricted Stock Units (RSUs): Shares of company stock that vest over a set period (e.g., 25% per year over four years). This can add significant value, especially if the company's stock performs well.
- Stock Options: The right to buy company stock at a predetermined price. These are more common in pre-IPO biotech startups and can be incredibly lucrative if the company is successful.
- Sign-On Bonus: In a competitive hiring market, companies often offer a one-time sign-on bonus to attract top talent. This can range from $5,000 to $25,000 or more, depending on the seniority of the role and the urgency of the need.
- Profit Sharing: Some companies offer a profit-sharing plan, where a portion of the company's profits is distributed among employees.
- Comprehensive Benefits: This is a critical, though non-cash, part of compensation. Top-tier employers offer:
- Excellent health, dental, and vision insurance with low employee premiums.
- A strong 401(k) or 403(b) retirement plan with a generous company match (e.g., 50% or 100% match up to 6% of your salary).
- Generous Paid Time Off (PTO), sick leave, and paid holidays.
- Parental leave, tuition reimbursement, and wellness programs.
When evaluating a job offer, it is crucial to look at the entire compensation package, not just the base salary. A role with a $130,000 base salary plus a 15% bonus and an annual RSU grant of $20,000 is significantly more lucrative than a role with a flat $140,000 salary and no bonus or equity.
Key Factors That Influence a Clinical Study Manager's Salary
While the national averages provide a useful benchmark, a CSM's actual salary is influenced by a confluence of factors. Understanding these variables is key to negotiating your worth and maximizing your earning potential throughout your career. This section, the most detailed in our guide, will break down each of these critical elements.
###
Level of Education
Your educational background forms the foundation of your career in clinical research and has a direct impact on your starting salary and long-term trajectory.
- Bachelor's Degree (BS/BA): A bachelor's degree in a life science (e.g., biology, chemistry, biochemistry), nursing (BSN), or a related health field is the standard minimum requirement. Professionals with a BSN are often highly valued as they bring direct patient care experience, which is invaluable for understanding site operations and patient perspectives.
- Master's Degree (MS/MPH/MSN): An advanced degree can provide a competitive edge and a salary premium. A Master of Science (MS) in Clinical Research, a Master of Public Health (MPH), or a Master of Science in Nursing (MSN) can lead to a higher starting salary, often by 5% to 15%. These degrees signal a deeper commitment to the field and provide specialized knowledge in areas like epidemiology, biostatistics, and research ethics, which are directly applicable to the CSM role.
- Doctoral Degree (PhD/PharmD/MD): While not required for most operational CSM roles, a doctorate can be a significant differentiator, particularly in scientifically complex therapeutic areas like oncology or gene therapy. A PhD can command a higher salary and may open doors to more strategic roles that bridge clinical operations and clinical science. A PharmD or MD who moves into an operational role is rare but can command a top-tier salary due to their deep clinical and scientific expertise.
- Professional Certifications: Certifications are a powerful way to validate your expertise and can lead to higher pay. The two most recognized certifications in the field are:
- Association of Clinical Research Professionals (ACRP): Offers certifications like ACRP-CP and ACRP-PM.
- Society of Clinical Research Associates (SoCRA): Offers the Certified Clinical Research Professional (CCRP®) credential.
Holding one of these certifications can demonstrate your knowledge of GCP and regulatory guidelines, making you a more attractive candidate and potentially adding a $5,000 to $10,000 premium to your salary, according to industry surveys.
###
Years of Experience
As illustrated in the previous section, experience is paramount. However, it's not just the number of years but the *quality* and *type* of experience that matter.
- The Classic Pathway (CRA to CSM): The most common trajectory is for a Clinical Research Associate (CRA) to be promoted to a CSM role after several years of experience. A CRA who has independently managed multiple high-performing sites, worked on complex studies, and taken on lead CRA responsibilities is well-positioned for the jump.
- 0-2 years (CRA I/II): Salary typically in the $70k - $100k range.
- 3-5 years (Senior CRA): Salary often $100k - $125k. This is the prime stage for transitioning to a CSM role, starting in the $110k - $125k range.
- Direct Study Management Experience: Your salary as a CSM will increase substantially as you build a track record of success.
- First Study Managed: The first time you lead a study from start to finish is a massive career milestone. Successfully delivering a study on time and on budget makes you significantly more marketable.
- Managing Complex Trials: Experience managing global trials, Phase III pivotal trials, or studies with challenging patient populations is a major salary driver. A CSM who has managed a 50-site, 15-country study is far more valuable than one who has only managed a 5-site, single-country Phase I study.
- NDA/BLA Submission Experience: Being part of a team that successfully submits a New Drug Application (NDA) or Biologics License Application (BLA) to the FDA is a career-defining achievement. CSMs with this experience are in high demand and can command salaries at the very top of the pay scale.
###
Geographic Location
Where you live and work has a massive impact on your salary, primarily due to variations in the cost of living and the concentration of pharmaceutical and biotech companies.
Major biotech and pharmaceutical hubs offer the highest salaries but also have a much higher cost of living.
High-Paying Metropolitan Areas:
- San Francisco Bay Area, CA: Often the highest-paying region in the US due to the dense cluster of innovative biotech companies. A CSM here can expect a salary 20-35% above the national average. A median salary could be $160,000 - $185,000.
- Boston/Cambridge, MA: Another top-tier hub with a mix of big pharma and cutting-edge biotech. Salaries are comparable to the Bay Area, typically 15-30% above average.
- San Diego, CA: A major life sciences cluster offering salaries that are roughly 10-20% above the national average.
- New York/New Jersey Area: The "pharma corridor" of New Jersey, combined with New York's growing biotech scene, makes this a high-paying region, often 10-25% above average.
- Raleigh-Durham (Research Triangle Park), NC: A major hub for CROs and pharmaceutical companies, offering strong salaries that are often 5-15% above the national average, but with a more moderate cost of living than the coastal hubs.
Average and Lower-Paying Regions:
Salaries tend to be closer to or slightly below the national average in areas with a lower cost of living and fewer life science companies. However, the purchasing power of that salary may be greater. States in the Midwest and some parts of the South will generally offer lower nominal salaries.
The Rise of Remote Work: The COVID-19 pandemic accelerated the trend of remote work in clinical operations. Many CSM roles are now fully remote. This has had a complex effect on salary. Some companies now pay based on the employee's location ("geo-neutral" pay is rare), while others have a national salary band. A remote role can be a significant financial advantage if you can secure a salary based on a high-cost hub while living in a lower-cost area.
###
Company Type & Size
The type of organization you work for is a major determinant of your compensation structure and overall work environment.
- Large Pharmaceutical Companies ("Big Pharma"):
- Salary: Generally offer the highest base salaries, robust annual bonuses, and excellent LTI (RSU) packages. They have structured salary bands and well-defined career ladders.
- Pros: Stability, excellent benefits, access to massive resources, opportunities to work on blockbuster drugs.
- Cons: Can be bureaucratic, slower-paced, and individual impact may feel diluted.
- Clinical Research Organizations (CROs):
- Salary: Highly competitive base salaries and bonuses, as CSMs (often called Project Managers at CROs) are their core business. Compensation is often heavily tied to project performance and client satisfaction.
- Pros: Exposure to a wide variety of therapeutic areas, sponsors, and study phases. Often faster-paced with rapid learning opportunities.
- Cons: Can be high-pressure with a focus on billable hours. Work-life balance can be a challenge.
- Biotechnology Companies ("Biotech"):
- Salary: This varies wildly. Well-funded, late-stage biotechs can compete directly with Big Pharma on salary and bonus.
- Equity: The big differentiator is equity. Pre-IPO or small-cap biotechs may offer a slightly lower base salary but compensate with a significant grant of stock options, which could be worthless or worth millions depending on the company's success. This is a high-risk, high-reward scenario.
- Pros: Innovative, fast-paced, entrepreneurial culture. Opportunity to make a huge impact and wear many hats.
- Cons: Less job security, tighter resources, and higher risk.
- Academic / Government / Non-Profit:
- Salary: Typically the lowest base salaries, often 15-30% below industry rates.
- Pros: Excellent work-life balance, generous retirement plans (pensions), and a focus on mission-driven, investigator-initiated research.
- Cons: Slower pace, limited resources, and lower overall earning potential.
###
Therapeutic Area of Specialization
The specific area of medicine in which you specialize can significantly influence your salary. Demand is highest for CSMs with experience in complex, high-investment therapeutic areas.
- Top Tier (Highest Demand & Salary):
- Oncology/Immuno-Oncology: The largest and most funded area of drug development. The complexity of study designs, patient populations, and novel therapies means experienced oncology CSMs are in extremely high demand and can command premium salaries.
- Rare Diseases / Orphan Drugs: These studies often involve small, geographically dispersed patient populations and complex logistics, requiring highly skilled managers.
- Cell & Gene Therapy: This cutting-edge field requires specialized knowledge of logistics (e.g., chain of custody for patient cells) and complex manufacturing processes. It is currently one of the highest-paying specializations.
- Mid Tier (Strong Demand):
- Neurology / Central Nervous System (CNS): Alzheimer's, Parkinson's, and other neurodegenerative disease trials are long and complex.
- Immunology/Rheumatology: Chronic inflammatory diseases are a major area of research.
- Cardiovascular & Metabolic Diseases: While some areas are more commoditized, novel therapies for heart failure or diabetes are still a major focus.
- Lower Tier (Standard Demand):
- Vaccines, Dermatology, Primary Care, Medical Devices: While still essential, the operational models for these studies can sometimes be less complex than those in oncology or rare diseases, which can be reflected in slightly more modest salary ranges.
###
In-Demand Skills
Beyond your background, the specific skills you cultivate can make you a more effective and higher-paid CSM.
- Hard Skills:
- Project Management Mastery: Proficiency in tools like MS Project, Smartsheet, or other project management software. Deep knowledge of risk management, budget forecasting, and resource planning.
- Vendor Management & Negotiation: The ability to negotiate contracts with CROs and other vendors can save a study millions of dollars, making this a highly valued skill.
- Data-Driven Decision Making: Comfort with clinical trial metrics, KPIs, and data visualization tools (like Tableau or Spotfire). The ability to interpret data to make proactive decisions is critical.
- Regulatory Expertise: Deep and up-to-date knowledge of FDA, EMA, and ICH-GCP regulations. Experience with regulatory submissions (NDA/BLA) is a huge plus.
- Soft Skills:
- Leadership & Influence: The ability to lead a cross-functional team where most members do not report directly to you. This requires influencing, motivating, and holding people accountable without formal authority.
- Exceptional Communication: Articulating complex information clearly to different audiences, from executive leadership to site coordinators.
- Problem-Solving & Critical Thinking: Clinical trials are unpredictable. The best CSMs are proactive problem-solvers who can think on their feet and make sound decisions under pressure.
- Resilience & Adaptability: The ability to manage stress, navigate setbacks, and adapt to the constantly changing landscape of a clinical trial.
Job Outlook and Career Growth

The career outlook for Clinical Study Managers is exceptionally bright. The demand for skilled professionals to manage the increasingly complex and global landscape of clinical trials is robust and expected to grow significantly in the coming decade.
### A High-Growth Profession
While the U.S. Bureau of Labor Statistics (BLS) does not have a separate category for "Clinical Study Manager," they are included within the broader category of "Medical and Health Services Managers." The data for this group is a strong indicator of the health of the profession.
According to the BLS Occupational Outlook Handbook, employment for Medical and Health Services Managers is projected to grow 28 percent from 2022 to 2032. This is described as "much faster than the average for all occupations." The BLS projects about 54,700 openings for these managers each year, on average, over the decade.
What's Driving This Growth?
- An Aging Population: As the large baby-boomer population ages, the incidence of chronic diseases like cancer, heart disease, and Alzheimer's is increasing, driving a greater need for new treatments and, consequently, more clinical trials.
- Scientific Advancement: Breakthroughs in genomics, personalized medicine, cell and gene therapy, and biologics are leading to a pipeline of innovative but highly complex new drugs. These trials require more sophisticated management than ever before.
- Globalization of Clinical Trials: Many large-scale trials are conducted globally to access diverse patient populations and speed up enrollment. This adds layers of regulatory and logistical complexity, increasing the need for skilled CSMs